Hepatitis C Rapid Test Device (WB/S/P)
HCV Rapid Test Device (WB/S/P)
INTENDED USE
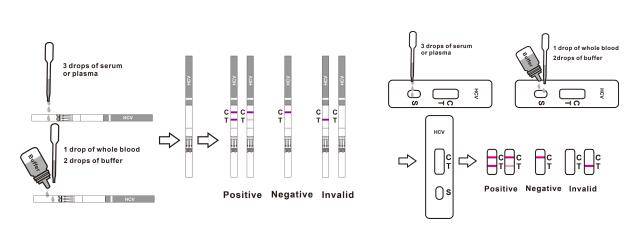
The HCV Rapid Test Cassette/Strip is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies to Hepatitis C Virus in Whole Blood/Serum/Plasma. It provides an aid in the diagnosis of infection with Hepatitis C Virus.
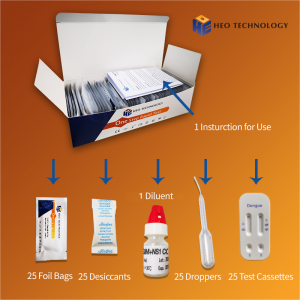
COMPONENT
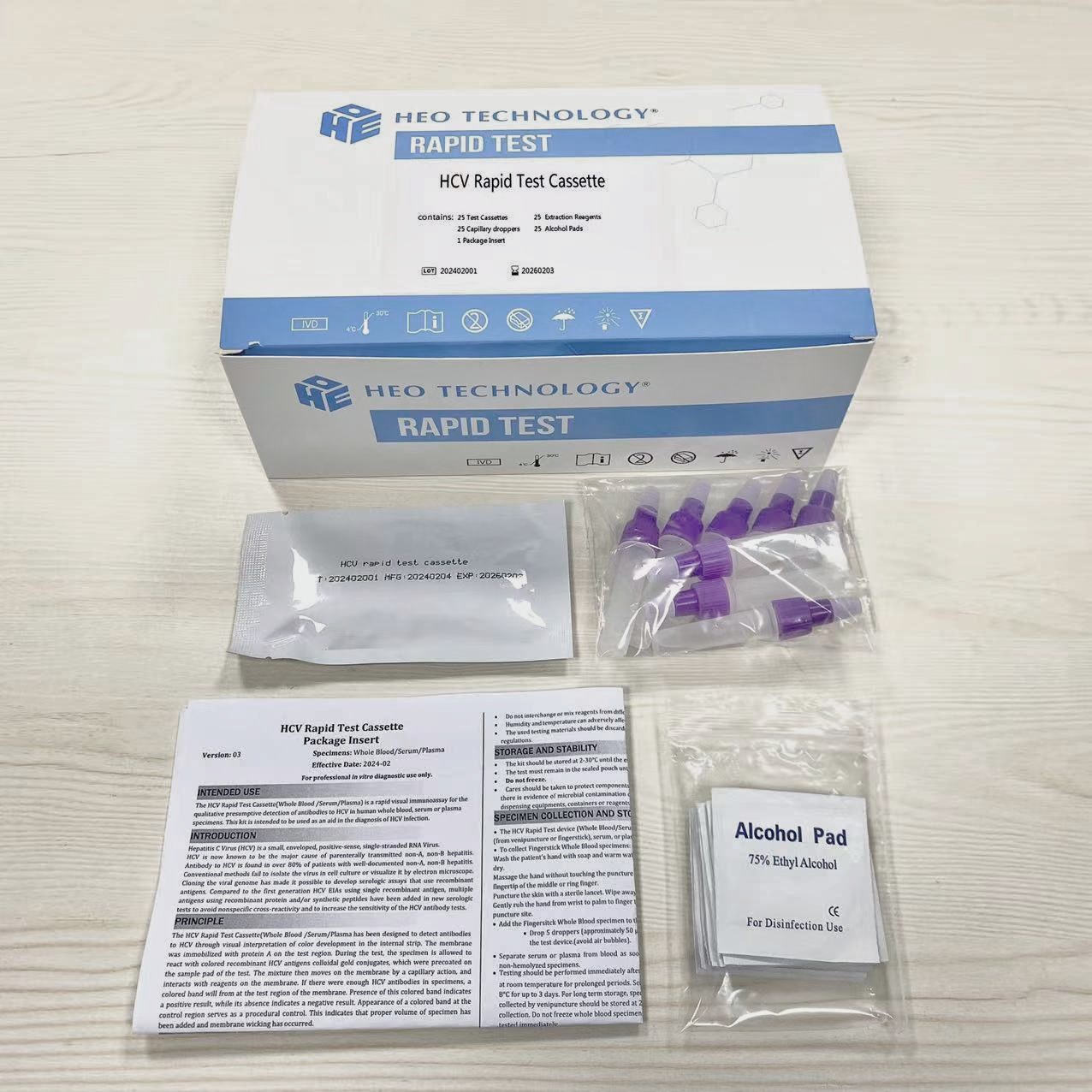
- Test cassette
- Package insert
- Buffer
- Dropper
STORAGE AND STABILITY
- The kit should be stored at 2‐30°C until the expiry date printed on the sealed pouch.
- The test must remain in the sealed pouch until use.
- Do not freeze.
- Cares should be taken to protect components in this kit from contamination. Do not use if there is evidence of microbial contamination or precipitation.
- Biological contamination of dispensing equipments, containers or reagents can lead to false results
PERFORMANCE CHARACTERISTICS
PRINCIPLE
The HCV Rapid Test Cassette/Strip is an immunoassay based on the principle of the double antigen-sandwich technique. During testing, a Whole Blood/Serum/Plasma specimen migrates upward by capillary action. The antibodies to HCV if present in the specimen will bind to the HCV conjugates. The immune complex is then captured on the membrane by the pre-coated recombinant HCV antigens, and a visible colored line will show up in the test line region indicating a positive result. If antibodies to HCV are not present or are present below the detectable level, a colored line will not form in the test line region indicating a negative result.
To serve as a procedural control, a colored line will always appear at the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.