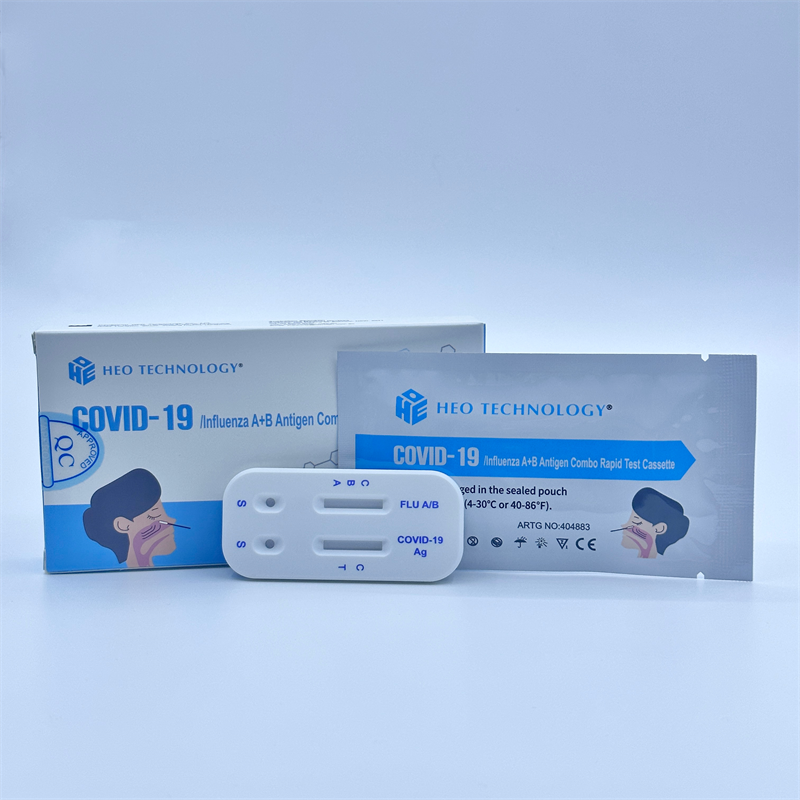
HK-MDD Certificate RTK Of COVID-19/Influenza A+B Antigen Combo Rapid Test
COVID-19/Influenza A+B Antigen Combo Rapid Test Cassette Austrilia ARTG No. 404883 HKMD No. 230344
INTENDED USE
The COVID-19/Influenza A+B Antigen Combo Rapid Test Cassette is a lateral flow immunoassay intended for the qualitative detection of SARSCoV-2, influenza A and influenza B viral nucleoprotein antigens in nasopharyngeal swab from individuals suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider. The COVID-19/Influenza A+B Antigen Combo Rapid Test Cassette is intended for use by trained clinical laboratory personnel specifically instructed and trained in vitro diagnostic procedures.
Product information
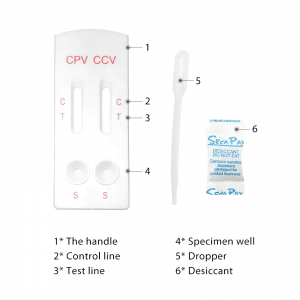
COMPONENT
Materials Provided Test Cassette: a test cassette includes the COVID-19 Antigen Test Strip and the Influenza A+B Test Strip, which are fixed inside a plastic device
· Extraction Reagent: Ampoule containing 0.4 mL of extraction reagent
· Sterilized Swab
· Extraction Tube
· Dropper Tip
· Work Station
· Package Insert
The quantity of tests was printed on the labeling. Materials required but not provided Timer
Operation steps and result interpretation