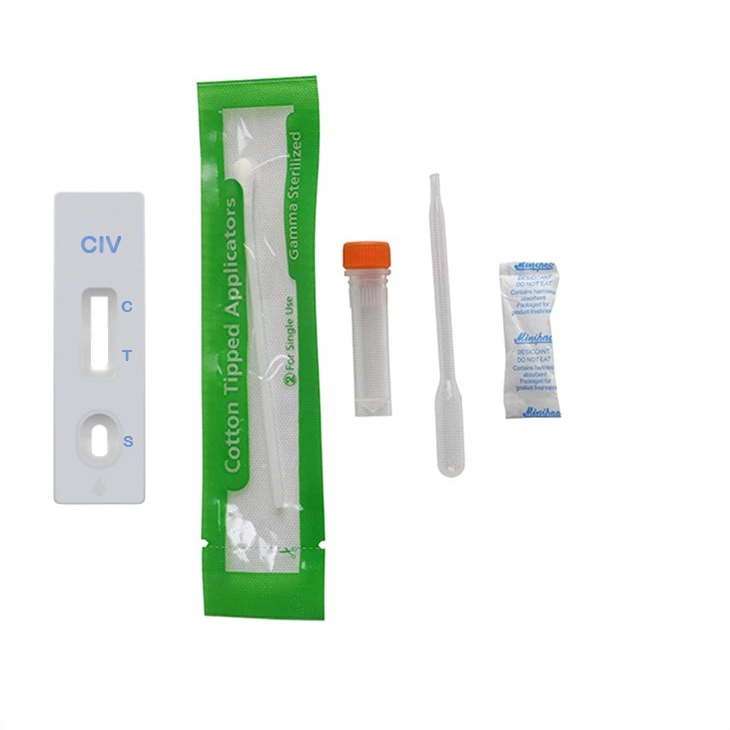
(CIV) Dog Influenza Virus Antigen Test Kit
Product name
(CIV Ag) Dog Influenza Virus Antigen Test Kit
Sample type : secretion samples such as eyelid and nasopharynx of the dog
Storage temperature
2°C - 30°C
Ingredients and Content
CIV Ag test card
Sampling cotton swabs (1/bag)
Eropper (1/bag)
Desiccant (1 bag/bag
Diluent (50 bottles/box)
Instruction (1 copy/box)
[Intended use]
The product is used for quickly detecting whether the dog is infected with the canine influenza virus through immunochromatographic test on respiratory secretion samples such as eyelid and nasopharynx of the dog
[Usage]
Read the IFU completely before testing, allow the test device and specimens to equilibrate to room temperature (15~25℃) prior to testing.
Method :
1. Samples were gently collected from the conjunctiva, nasal cavity, or oral cavity of the animal using a cotton swab. Immediately insert the cotton swab into the sample tube containing the buffer and mix the solutions so that the specimen dissolves in as much solution as possible. Given the uncertainty regarding the site of detoxification in animals, it is recommended that samples be collected from multiple sites during clinical testing and mixed in sample dilutions to avoid detection leakage.
2. Take a piece of CIV test card pocket and open, remove the test card, and place it horizontally on the operator's platform.
3. Pipette the sample solution to be tested into the sample well S and add 3-4 drops (approximately 100μL).
4. Observe the result within 5-10 minutes, and the result is invalid after 15 minutes.
[Result judgment]
* Positive (+): The wine red bands of control line C and detection line T indicated that the sample contained foot-and- mouth disease type A antibody.
* Negative (-): No color developed on the test T-ray, indicating that the sample did not contain foot-and-mouth disease type A antibody.
* Invalid: No QC Line C or Whiteboard present indicating incorrect procedure or invalid card. Please retest.
[Precautions]
1. Please use the test card within the guarantee period and within one hour after opening:
2. When testing to avoid direct sunlight and electric fan blowing;
3. Try not to touch the white film surface in the center of the detection card;
4. Sample dropper cannot be mixed, so as to avoid cross contamination;
5. Do not use sample diluent that is not supplied with this reagent;
6. After the use of detection card should be regarded as microbial dangerous goods processing;
[Application limitations]
This product is an immunological diagnostic kit and is only used to provide qualitative test results for clinical detection of pet diseases. If there is any doubt about the test results, please use other diagnostic methods (such as PCR, pathogen isolation test, etc.) to make further analysis and diagnosis of the detected samples. Consult your local veterinarian for pathological analysis.
[Storage and expiration]
This product should be stored at 2℃–40℃ in a cool, dry place away from light and not frozen; Valid for 24 months.
See the outer package for the expiration date and batch number.