HCG Pregnancy Rapid Test Cassette
HCG Pregnancy Rapid Test Cassette
[Background]
The hCG Pregnancy Midstream Test (Urine) is a rapid chromatographic immunoassay for the qualitative detection of human chorionic gonadotropin in urine to aid in the early detection of pregnanc
[Usage]
Please read the operating instructions carefully before testing, and restore the test card and the sample to be tested to room temperature of 2–30℃.
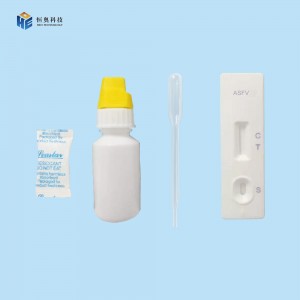
1. Bring the pouch to room temperature (15-30℃) before opening it. Remove the cassette from the sealed pouch and use it as soon as possible.
2. Place the cassette on a clean and level surface. Hold the dropper vertically and transfer 3 full drops of urine (approximately 120ul) to the specimen well of the cassette, and then start the timer. Avoid trapping air bubbles in the specimen well. See illustration below.
3. Wait for the colored line(s) to appear. Read the result at 3 minutes when testing a urine specimen.
NOTE: A low hCG concentration might result in a weak line appearing in the test line region (T) after an extended period of time; therefore, do not interpret the result after 10 minutes
[Result judgment]
POSITIVE: Two distinct red lines appear*. One line should be in the control line region (C) and another line should be in the test line region (T).
NOTE: The intensity of the colour in the test line region (T) may vary depending on the concentration of hCG present in the specimen. Therefore, any shade of colour in the test line region (T) should be considered positive.
NEGATIVE: One red line appears in the control line region (C). No apparent coloured line appears in the test line region (T).
INVALID: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test. If the problem persists, discontinue using the test kit immediately and contact your local supplier.
[Application limitations]
1. The hCG Pregnancy Midstream Test (Urine) is a preliminary qualitative test, therefore, neither the quantitative value nor the rate of increase in hCG can be determined by this test.
2. Very dilute urine specimens as indicated by a low specific gravity, may not contain representative levels of hCG. If pregnancy is still suspected, a first morning urine specimen should be collected 48 hours later and tested.
3. Very low levels of hCG (less than 50 mIU/mL) are present in urine specimens shortly after implantation. However, because a significant number of first trimester pregnancies terminate for natural reasons,5 a test result that is weakly positive should be confirmed by retesting with a first morning urine specimen collected 48 hours later.
4. This test may produce false positive results. A number of conditions other than pregnancy, including trophoblastic disease and certain non-trophoblastic neoplasms including testicular tumours, prostate cancer, breast cancer and lung cancer can cause elevated levels of hCG.6,7 Therefore, the presence of hCG in urine should not be used to diagnose pregnancy unless these conditions have been ruled out.
5. This test may produce false negative results. False negative results may occur when the levels of hCG are below the sensitivity level of the test. When pregnancy is still suspected, a first morning urine specimen should be collected 48 hours later and tested. In cases where pregnancy is suspected and the test continues to produce negative results, see a physician for further diagnosis.
6. This test provides a presumptive diagnosis for pregnancy. A confirmed pregnancy diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
[Storage and expiration]
This product should be stored at 2 ℃–30℃ dry place away from light and not frozen; Valid for 24 months. See the outer package for the expiration date and batch number.