Foot-and-mouth Disease Virus (FMDV) NSP Antibody Test Kit
[Background]
The product can quickly determine the condition of the foot-and-mouth disease virus NSP antibody in animals through immunochromatographic experiments on serum or plasma samples and detection of the foot-and-mouth disease virus NSP specific antibody, thereby providing reference for clinical diagnosis of foot-and-mouth disease virus type O.
[Detection principle]
This product uses rapid immunochromatography for the detection of foot-and-mouth disease virus NSP antibodies. Aft that sample is added into the sample adde hole of the detection card, if the foot-and-mouth disease virus NSP antibody exists in the sample, the antibody can specifically combine with the foot-and-mouth disease virus NSP antigen marked by colloidal gold to form a compound which moves along the chromatographic membrane and is capture by the protein pre-coated on the chromatographic membrane to form a wine red detection line at the position t of the clam shell. In the absence of FMDV type O antibody in the sample, no visible line was formed at the T position. In addition, a C-line was also designed in this system to verify the effectiveness of the experiment. The line shall be coloured either negative or positive, otherwise it shall be declared invalid.
[Product composition]
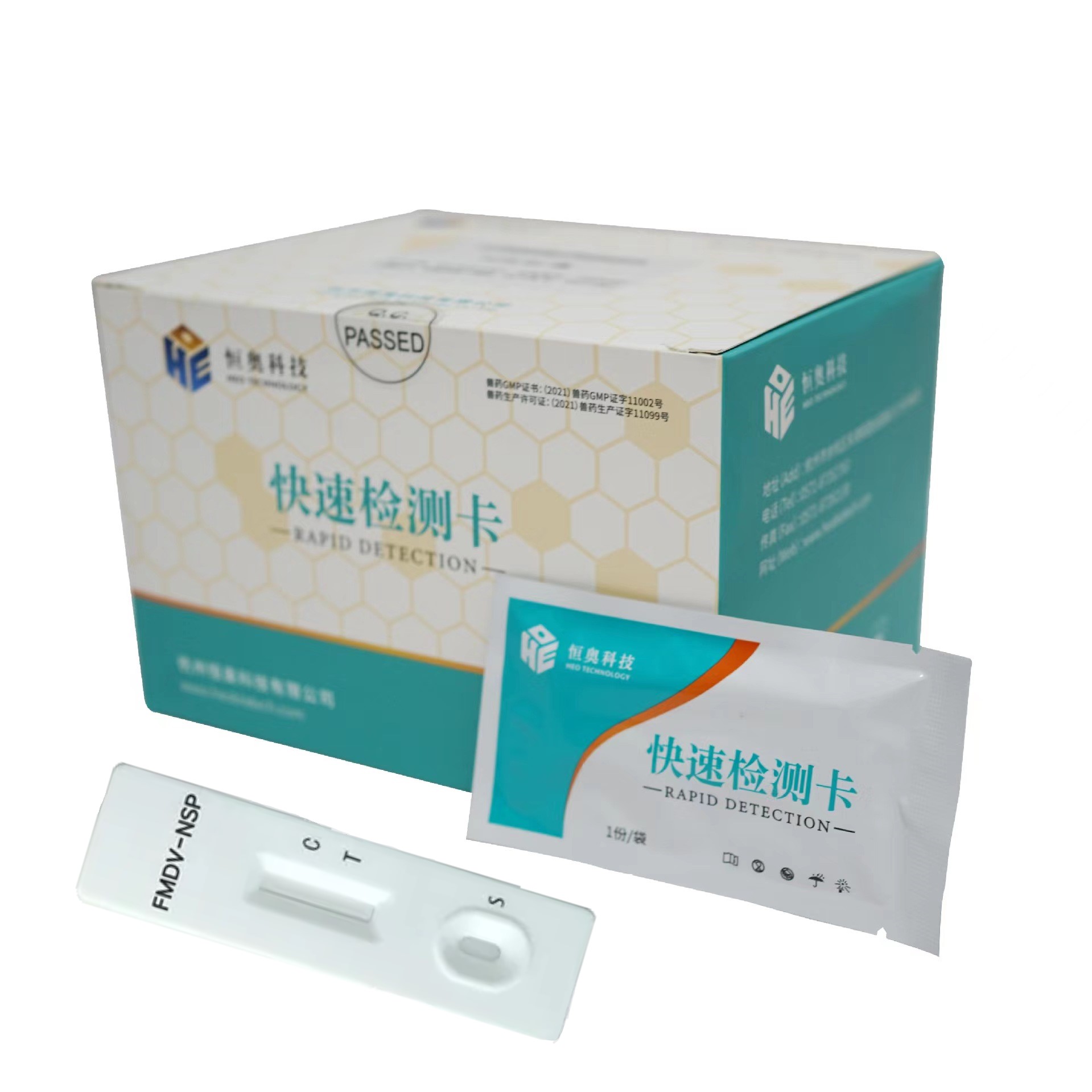
Foot-and-mouth disease virus type O antibody test kit (50 bags/ box)
Dropper ( 1 pc/bag)
Desiccant (1pc/bag)
Instruction ( 1 pc/box)
[Usage]
Please read the operating instructions carefully before testing, and restore the test card and the sample to be tested to room temperature of 15–25℃.
1. Fresh whole blood was collected, serum was separated by standing, or plasma samples were obtained by centrifugation, and samples were ensured not to be cloudy or precipitated.
2. Take out a piece of test card pocket and tear open, take out the test card, make it level on the operation platform.
3. Into sample well "S", add 2-3 drops (approximately 70-100 mL) of sample.
4. Observations within 5- 10 minutes, invalid after 15 minutes.
[Result judgment]
* Positive (+): The wine red bands of control line C and detection line T indicated that the sample contained foot-and- mouth disease type A antibody.
* Negative (-): No color developed on the test T-ray, indicating that the sample did not contain foot-and-mouth disease type A antibody.
* Invalid: No QC Line C or Whiteboard present indicating incorrect procedure or invalid card. Please retest.
[Precautions]
1. Please use the test card within the guarantee period and within one hour after opening:
2. When testing to avoid direct sunlight and electric fan blowing;
3. Try not to touch the white film surface in the center of the detection card;
4. Sample dropper cannot be mixed, so as to avoid cross contamination;
5. Do not use sample diluent that is not supplied with this reagent;
6. After the use of detection card should be regarded as microbial dangerous goods processing;
[Application limitations]
This product is an immunological diagnostic kit and is only used to provide qualitative test results for clinical detection of pet diseases. If there is any doubt about the test results, please use other diagnostic methods (such as PCR, pathogen isolation test, etc.) to make further analysis and diagnosis of the detected samples. Consult your local veterinarian for pathological analysis.
[Storage and expiration]
This product should be stored at 2 ℃–40℃ in a cool, dry place away from light and not frozen; Valid for 24 months. See the outer package for the expiration date and batch number.