Wholesale Price China Coronavirus Test - COVID-19 Neutralizing Antibody Rapid Test Cassette (Colloidal gold) – HEO
Wholesale Price China Coronavirus Test - COVID-19 Neutralizing Antibody Rapid Test Cassette (Colloidal gold) – HEO Detail:
The COVID-19 Neutralizing Antibody Rapid Test Cassette(Colloidal Gold) is a rapid chromatographic immunoassay for the qualitative detection of neutralizing antibodies to COVID-19 in human whole blood, serum, or plasma as an aid in the diagnosis of the presence of neutralizing antibodies to COVID-19 .

The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
The COVID-19 Neutralizing Antibody Rapid Test Cassette(Colloidal Gold) is a rapid test that utilizes a combination of S-RBD antigen coated colored particles for the detection of neutralizing antibodies to COVID-19 in human whole blood, serum, or plasma.

The COVID-19 Neutralizing Antibody Rapid Test Cassette(Colloidal Gold) is a qualitative membrane based immunoassay for the detection of neutralizing antibodies to COVID-19 in whole blood, serum or plasma. The membrane is pre-coated with Angiotensin I Converting Enzyme 2 (ACE2) on the test line region of the strip. During testing, the whole blood, serum or plasma specimen reacts with S-RBD conjugated colloid gold. The mixture migrates upward on the membrane chromatographically by capillary action to react with ACE2 on the membrane and generate a colored line. Presence of this colored line indicates a negative result, while its absence indicates a positive result. To serve as a procedural control, a colored line will always change from Blue to Red in the control line region, indicating that the proper volume of specimen has been added and membrane wicking has occurred.

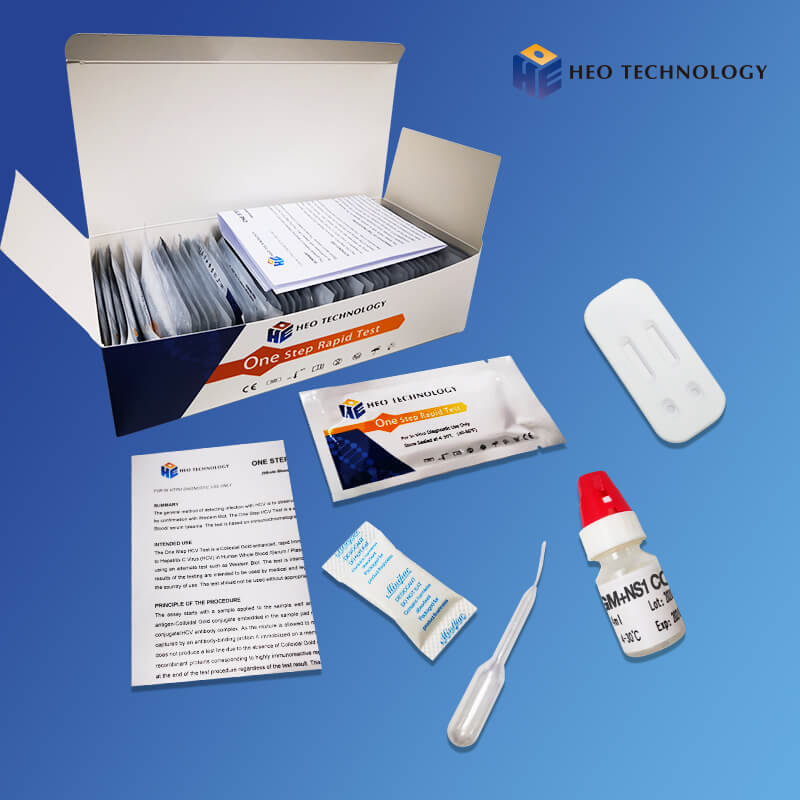
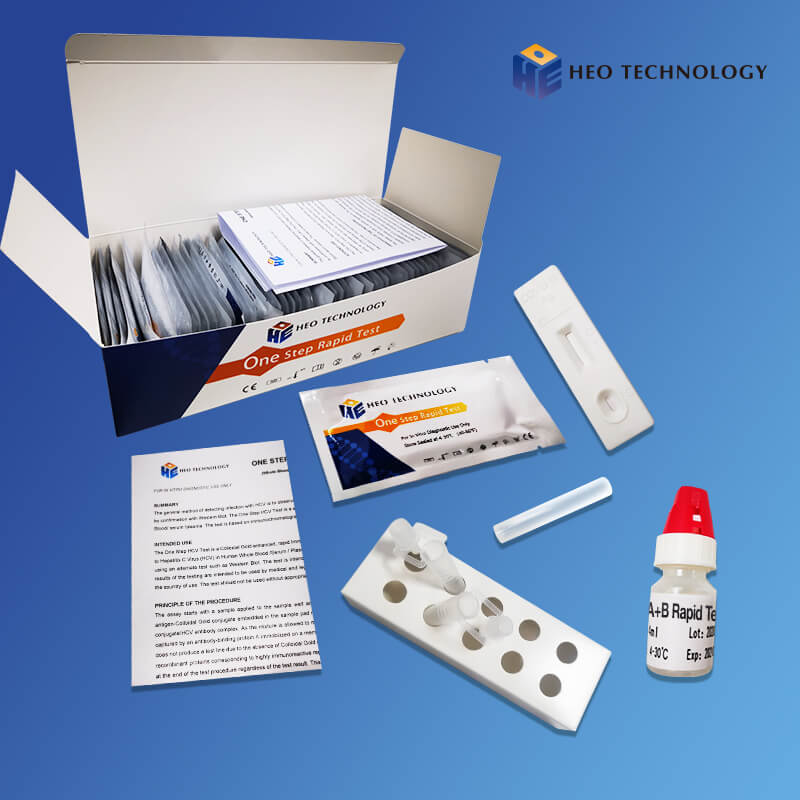
| Individually packed test devices | Each device contains a strip with colored conjugates and reactive reagents pre-spreaded at the corresponding regions |
| Disposable pipettes | For adding specimens use |
| Buffer | Phosphate buffered saline and preservative |
| Package insert | For operation instruction |

Materials Provided
| ●Test devices | ●Droppers |
| ●Buffer | ●Package insert |
Materials Required But Not Provided
| ●Specimen collection containers | ●Timer |
| ●Centrifuge |

1. For professional in vitro diagnostic use only.
2. Do not use after the expiration date indicated on the package. Do not use the test if the foil pouch is damaged. Do not reuse tests.
3. The extraction reagent solution contains a salt solution if the solution contacts the skin or eye, flush with copious amounts of water.
4. Avoid cross-contamination of specimens by using a new specimen collection container for each specimen obtained.
5. Read the entire procedure carefully prior to testing.
6. Do not eat, drink or smoke in the area where the specimens and kits are handled. Handle all specimens as if they contain infectious agents. Observe established precautions against microbiological hazards throughout the procedure and follow standard procedures for the proper disposal of specimens. Wear protective clothing such as laboratory coats, disposable gloves and eye protection when specimens are assayed.
7. If infection with a novel coronaviruses is suspected based on current clinical and epidemiological screening criteria recommended by public health authorities, specimens should be collected with appropriate infection control precautions for novel coronaviruses and sent to state or local health department for testing. Viral culture should not be attempted in these cases unless a BSL 3+ is available to receive and culture specimens.
8. Do not interchange or mix reagents from different lots.
9. Humidity and temperature can adversely affect results.
10. Used testing materials should be discarded in accordance with local regulations.

1. The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
2. The test must remain in the sealed pouch until use.
3. Do not freeze.
4. Care should be taken to protect the components of the kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipment, containers or reagents can lead to false results.

Consider any materials of human origin as infectious and handle them using standard biosafety procedures.
Capillary Whole Blood
Wash the patient’s hand then allow to dry. Massage the hand without touching the puncture. Puncture the skin with a sterile lancet. Wipe away the first sign of blood. Gently rub the hand from wrist to palm to finger to form a rounded drop of blood over the puncture site. Add the Fingerstick Whole Blood specimen to the test device by using a capillary tube or hanging drops.
venous Whole Blood:
Collect blood specimen into a lavender, blue or green top collection tube (containing EDTA, citrate or heparin, respectively in Vacutainer®) by veinpuncture.
Plasma
Collect blood specimen into a lavender, blue or green top collection tube (containing EDTA, citrate or heparin, respectively in Vacutainer®) by veinpuncture. Separate the plasma by centrifugation. Carefully withdraw the plasma into new pre-labeled tube.
Serum
Collect blood specimen into a red top collection tube (containing no anticoagulants in Vacutainer®) by veinpuncture. Allow the blood to clot. Separate the serum by centrifugation. Carefully withdraw the serum into a new pre-labeled tube.
Test specimens as soon as possible after collecting. Store specimens at 2°C-8°C if not tested immediately.
Store specimens at 2°C-8°C up to 5 days. The specimens should be frozen at -20°C for longer storage.
Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing. Do not use samples demonstrating gross lipemia, gross hemolysis or turbidity in order to avoid interference on result interpretation.

Bring the specimen and test components to room temperature Mix the specimen well prior to assay once thawed. Place the test device on a clean, flat surface.
For capillary whole blood sample:
To use a capillary tube: Fill the capillary tube and transfer approximately 50µL (or 2 drops) of fingerstick whole blood specimen to the specimen well (S) of the test device, then add 1 drop (about 30 µL) of Sample Diluent immediately into the sample well.
For whole blood sample:
Fill the dropper with the specimen then transfer 2 drops (about 50 µL) of specimen into the sample well. Making sure that there are no air bubbles. Then transfer 1 drop (about 30 µL) of Sample Diluent immediately into the sample well.
For Plasma/ Serum sample:
Fill the dropper with the specimen then transfer 1 drop (about 25 µL) of specimen into the sample well. Making sure that there are no air bubbles. Then transfer 1 drop (about 30 µL) of Sample Diluent immediately into the sample well.
Set up a timer. Read the result at 15 minutes. Don’t read result after 20 minutes. To avoid confusion, discard the test device after interpreting the result

|
|
Only one colored band appears in the control region (C). No apparent colored band appears in the test region (T). |
|
|
Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). |
| *NOTE: The intensity of the color in the test line region will vary depending on the concentration of neutralizing antibodies to COVID-19 in the specimen. Therefore, any shade of color in the test line region should be considered negative. | |
|
|
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be disgarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. |

1. Internal Control: This test contains a built-in control feature, the C band. The C line develops after adding specimen and sample diluent. Otherwise, review the whole procedure and repeat test with a new device.
2. External Control: Good Laboratory Practice recommends using the external controls, positive and negative (provided upon request), to assure the proper performing of the assay.
Product detail pictures:

Related Product Guide:
We attempt for excellence, company the customers", hopes to be the top cooperation team and dominator company for personnel, suppliers and customers, realizes price share and continual marketing for Wholesale Price China Coronavirus Test - COVID-19 Neutralizing Antibody Rapid Test Cassette (Colloidal gold) – HEO , The product will supply to all over the world, such as: Serbia, Argentina, Korea, Our staffs are adhering to the "Integrity-based and Interactive Development" spirit, and the tenet of "First-class Quality with Excellent Service". According to the needs of every customer, we provide customized & personalized services to help customers achieve their goals successfully. Welcome clients from home and abroad to call and inquire!
The company account manager has a wealth of industry knowledge and experience, he could provide appropriate program according our needs and speak English fluently.