Super Lowest Price Nsi Dengue Test - Influenza A+B Rapid Test Cassette – HEO
Super Lowest Price Nsi Dengue Test - Influenza A+B Rapid Test Cassette – HEO Detail:

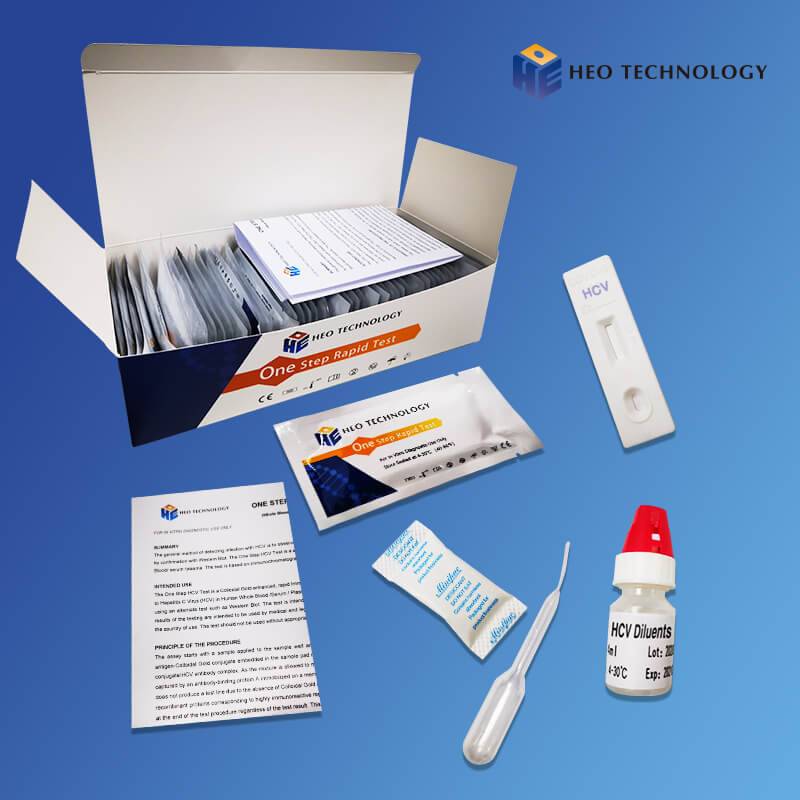
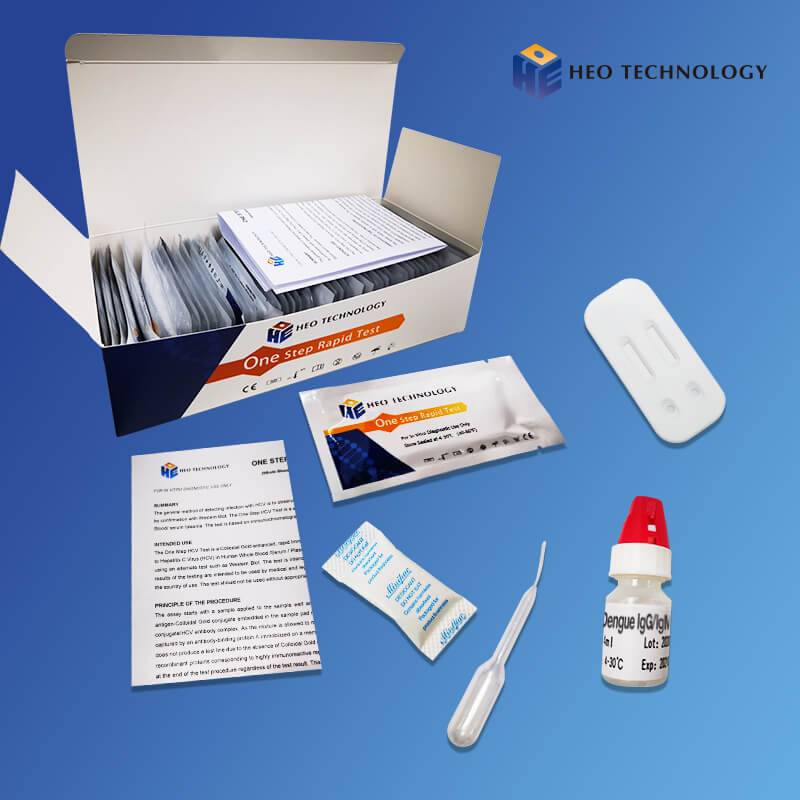
Influenza A+B Rapid Test Cassette
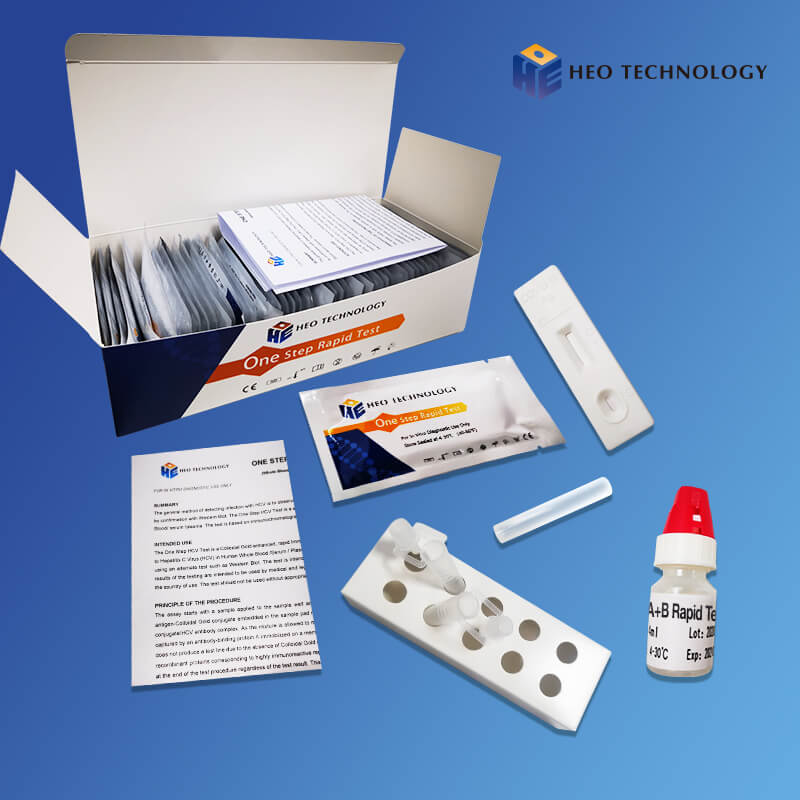

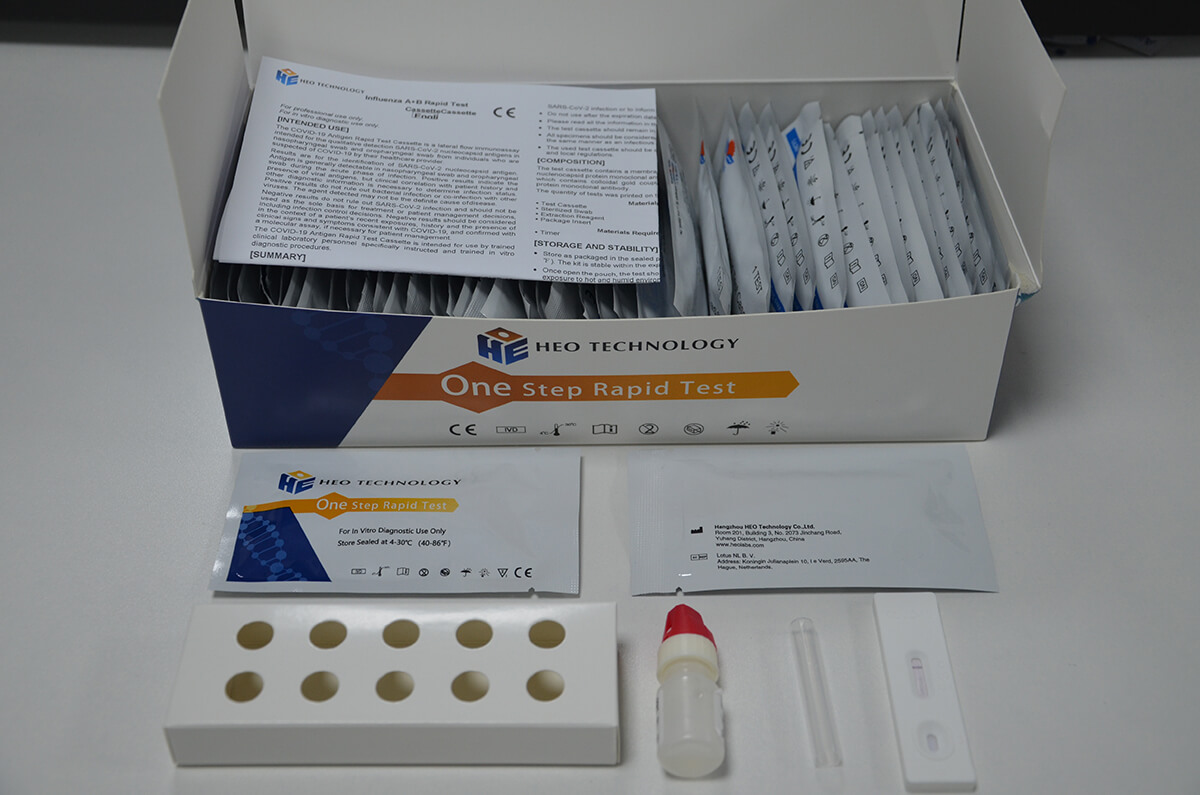


[INTENDED USE]
The Influenza A+B Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of influenza A and B viral antigens form throat swabs and nasopharyngeal swab specimens. The test is intended for use as an aid in the rapid differential diagnosis of acute influenza type A and type B virus infection.
PRINCIPLE
The Influenza A+B Rapid Test Cassette detects influenza A and B viral antigens through visual interpretation of color development on the strip. Anti-influenza A and B antibodies are immobilized on the test region A and B of the membrane respectively. During testing, the extracted specimen reacts with anti- influenza A and B antibodies conjugated to colored particles and precoated onto the sample pad of the test. The mixture then migrates through the membrane by capillary action and interacts with reagents on the membrane. If there is sufficient influenza A and B viral antigens in the specimen, colored band(s) will form at the according test region of the membrane. The presence of a colored band in the A and/or B region indicates a positive result for the particular viral antigens, while its absence indicates a negative result. The appearance of a colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking has occurred.
STORAGE AND STABILITY
1.The kit should be stored at 2-30°C until the expiry date printed on the sealed pouch.
2.The test must remain in the sealed pouch until use.
3.Do not freeze.
4.Care should be taken to protect the components of the kit from contamination. Do not use if there is evidence of microbial contamination or precipitation. Biological contamination of dispensing equipment, containers or reagents can lead to false results.
PROCEDURE
Bring tests, specimens, and/or controls to room temperature (15-30°C) before use.
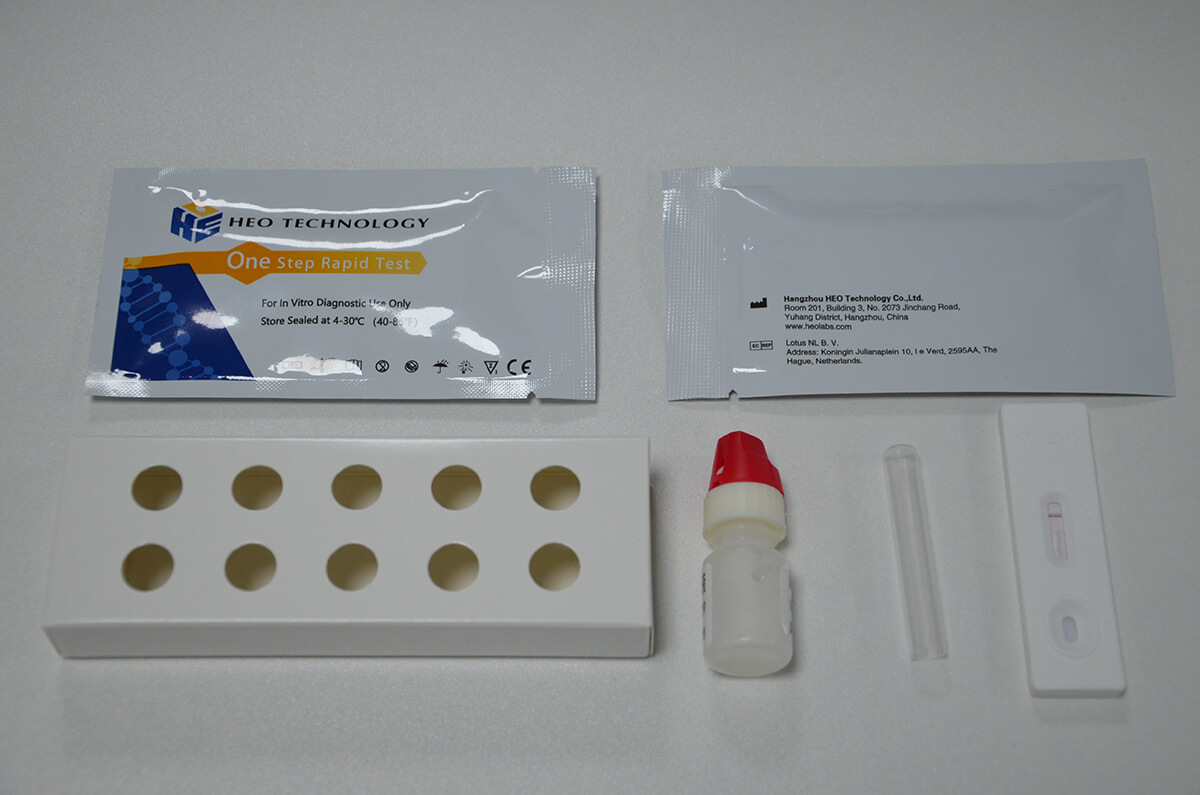
1.Remove the test from its sealed pouch, and place it on a clean, level surface. Label the Cassette with patient or control identification. For best results, the assay should be performed within one hour.
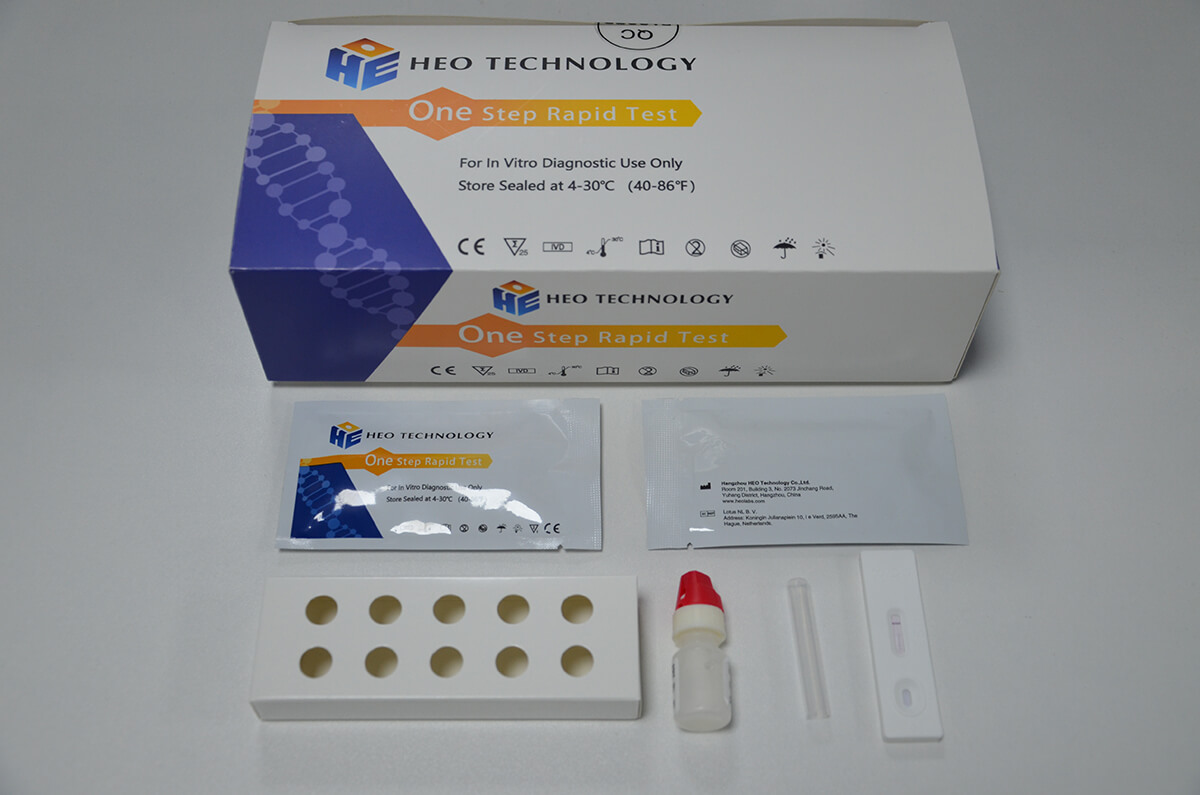
2.Gently mix Extraction reagent solution. Add 6 drops of the Extraction Solution into the Extraction tube.
3.Place the patient swab specimen into the Extraction Tube. Roll the swab at least 10 times while pressing the swab against the bottom and side of the Extraction Tube. Roll the swab head against the inside of the Extraction Tube as you remove it. Try to release as much liquid as possible. Dispose of the used swab in accordance with your biohazard waste disposal protocol.
4.Put on the tube tip, then add 4 drops of extracted sample into the sample well. Do not handle or move the Test Cassette until the test is complete and ready for reading.
5.As the test begins to work, color will migrate across the membrane. Wait for the colored band(s) to appear. The result should be read at 10 minutes. Do not interpret the result after 20 minutes.
NTERPRETATION OF RESULTS
Allow the test cassette and specimens to equilibrate to temperature (15-30℃ or 59-86℉) prior to testing
1. Remove the test cassette from the sealed pouch.
2. Reverse the specimen extraction tube, Holding the specimen extraction
tube upright, transfer 3 drops (approximately 100μl) to the specimen
well(S) of the test cassette, then start the timer. See illustration below.
Wait for colored lines to appear. Interpret the test results in 15 minutes. Do not read results after 20 minutes.
LIMITATIONS OF THE TEST
1.The Flu A+B Rapid Test Cassette is for professional in vitro diagnostic use, and should only be used for the qualitative detection of influenza A and/or B.
2.The etiology of respiratory infection caused by microorganisms other than influenza A or B virus will not be established with this test. The Flu A+B Rapid Test Cassette is capable of detecting both viable and non- viable influenza particles. The performance of the Flu A+B Rapid Test Cassette depends on antigen load and may not correlate with cell culture performed on the same specimen.
3.If the test result is negative and clinical symptoms persist, additional testing using other clinical methods is recommended. A negative result does not at anytime rule out the presence of influenza A and/or B viral antigens in specimen, as they may be present below the minimum detection level of the test. As with all diagnostic tests, a confirmed diagnosis should only be made by a physician after all clinical and laboratory findings have been evaluated.
4.The validity of Flu A+B Rapid Test Cassette has not been proven for identification or confirmation of cell culture isolates.
5.Inadequate or inappropriate specimen collection, storage, and transport may yield false negative test result.
6.Although this test has been shown to detect cultured avian influenza viruses, including avian influenza A subtype H5N1 virus, the performance characteristics of this test with specimens from humans infected with H5N1 or other avian influenza viruses are unknown.
7.Performance characteristics for influenza A were established when influenza A/H3 and A/H1 were the predominant influenza A viruses in circulation. When other influenza A viruses are emerging, performance characteristics may vary.
8.Children tend to shed virus for longer periods of time than adults, which may result in differences in sensitivity between adults and children.
9.Positive and negative predictive values are highly dependent on prevalence. False positive test results are more likely during periods of low influenza activity when prevalence is moderate to low.
NOTE:
1.The intensity of color in the test region (A/B) may vary depending on the concentration of analyses present in the specimen. Therefore, any shade of color in the test region (A/B) should be considered positive. Please note that this is a qualitative test only, and cannot determine the concentration of analytes in the specimen.
2.Insufficient specimen volume, incorrect operating procedure or expired tests are the most likely reasons for control band failure.
Product detail pictures:


Related Product Guide:
We strive for excellence, services the customers", hopes to be the top cooperation team and dominator business for personnel, suppliers and prospects, realizes benefit share and continual promotion for Super Lowest Price Nsi Dengue Test - Influenza A+B Rapid Test Cassette – HEO , The product will supply to all over the world, such as: Mauritius, Algeria, Nairobi, Insisting on the high quality generation line management and customers expert assistance, we now have designed our resolution to offer our buyers using the to start with amount getting and just after services practical experience. Maintaining the prevailing friendly relations with our buyers, we however innovate our solution lists all of the time to satisfy the brand new demands and adhere to the most up-to-date development of the market in Malta. We are ready to face the worries and make the improve to understand all the possibilities in international trade.
In China, we have many partners, this company is the most satisfying to us, reliable quality and good credit, it is worth appreciation.