Hot sale Factory Dengue Ns1 Test Device - ONE STEP HCV TEST (Whole Blood/Serum/Plasma) – HEO
Hot sale Factory Dengue Ns1 Test Device - ONE STEP HCV TEST (Whole Blood/Serum/Plasma) – HEO Detail:
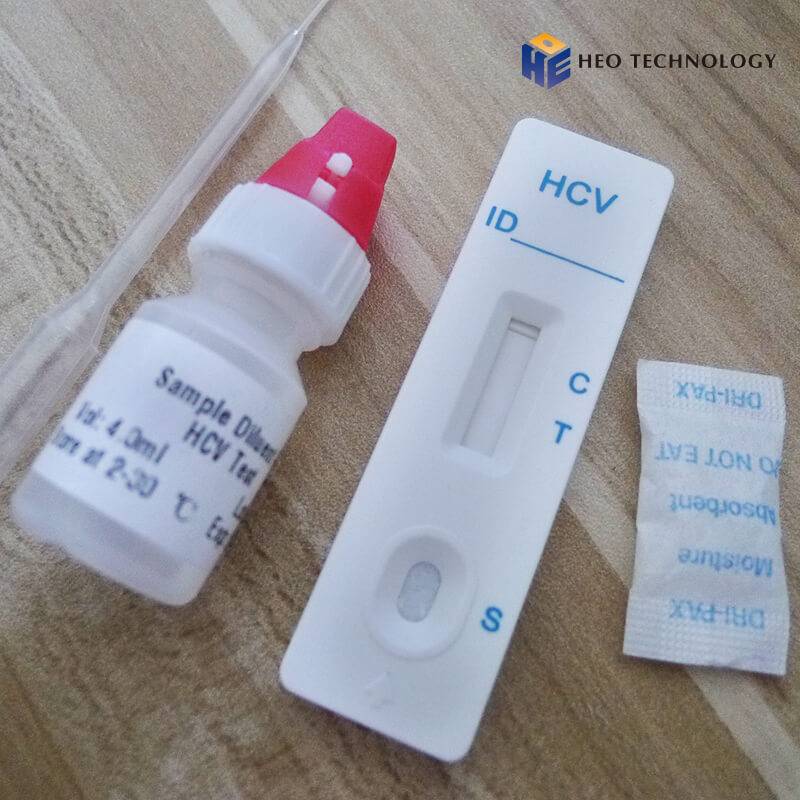
ONE STEP HCV TEST (Whole Blood/Serum/Plasma)


SUMMARY
The general method of detecting infection with HCV is to observe the presence of antibodies to the virus by an EIA method followed by confirmation with Western Blot. The One Step HCV Test is a simple, visual qualitative test that detects antibodies in human Whole Blood/ serum /plasma. The test is based on immunochromatography and can give a result within 15 minutes.
INTENDED USE
The One Step HCV Test is a Colloidal Gold enhanced, rapid Immunochromatoraphic Assay for the qualitative detection of antibodies to Hepatitis C Virus (HCV) in Human Whole Blood /Serum / Plasma. This test is a screening test and all positives must be confirmed using an alternate test such as Western Blot. The test is intended for Healthcare Professional use only. Both the testing and the results of the testing are intended to be used by medical and legal professionals only, unless otherwise authorized by regulation in the country of use. The test should not be used without appropriate supervision.
PRINCIPLE OF THE PROCEDURE
The assay starts with a sample applied to the sample well and the addition of the provided sample diluent immediately. HCV antigen-Colloidal Gold conjugate embedded in the sample pad reacts with the HCV antibody present in serum or plasma, forming conjugate/HCV antibody complex. As the mixture is allowed to migrate along the test strip, the conjugate/HCV antibody complex is captured by an antibody-binding protein A immobilized on a membrane forming a colored band in the test region. A negative sample does not produce a test line due to the absence of Colloidal Gold conjugate/HCV antibody complex. The antigens used in the test are recombinant proteins corresponding to highly immunoreactive regions of HCV. A colored control band in the control region appears at the end of the test procedure regardless of the test result. This control band is the result of Colloidal Gold conjugate binding to an anti-HCV antibody immobilized on the membrane. The control line indicates that the Colloidal Gold conjugate is functional. The absence of the control band indicates that the test is invalid.
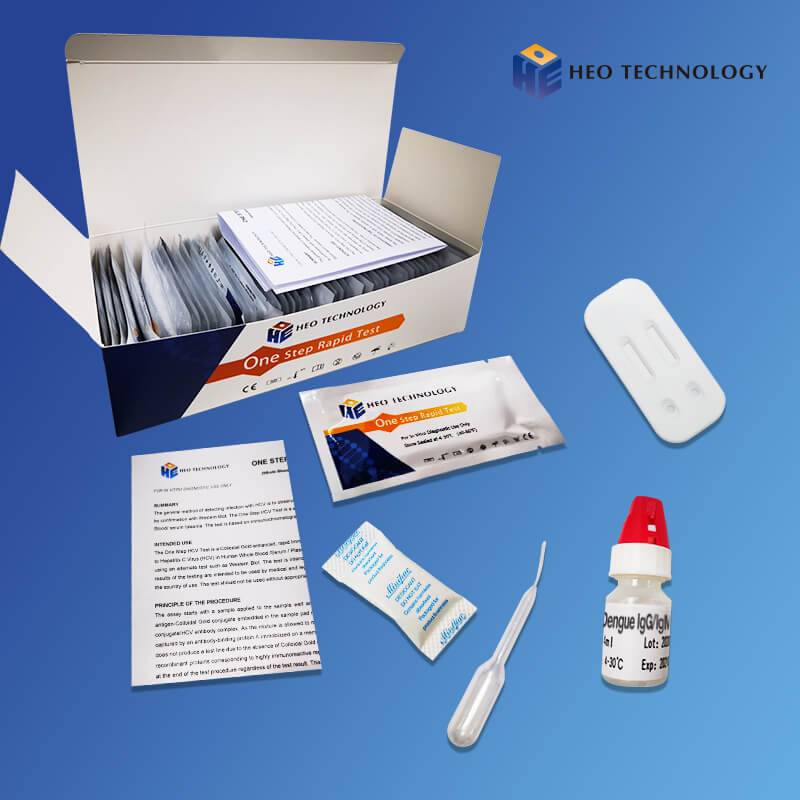
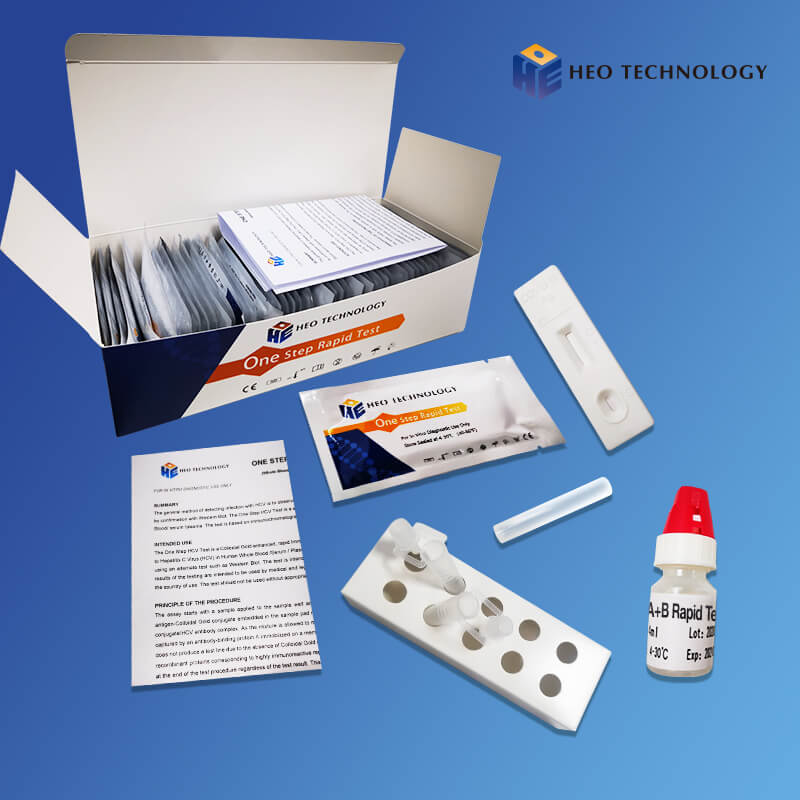
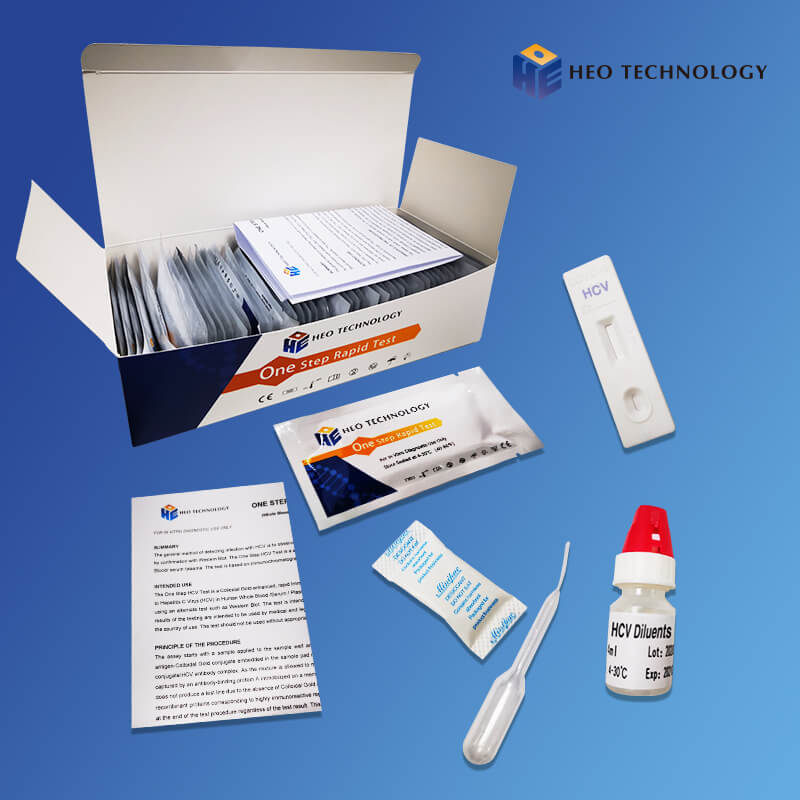
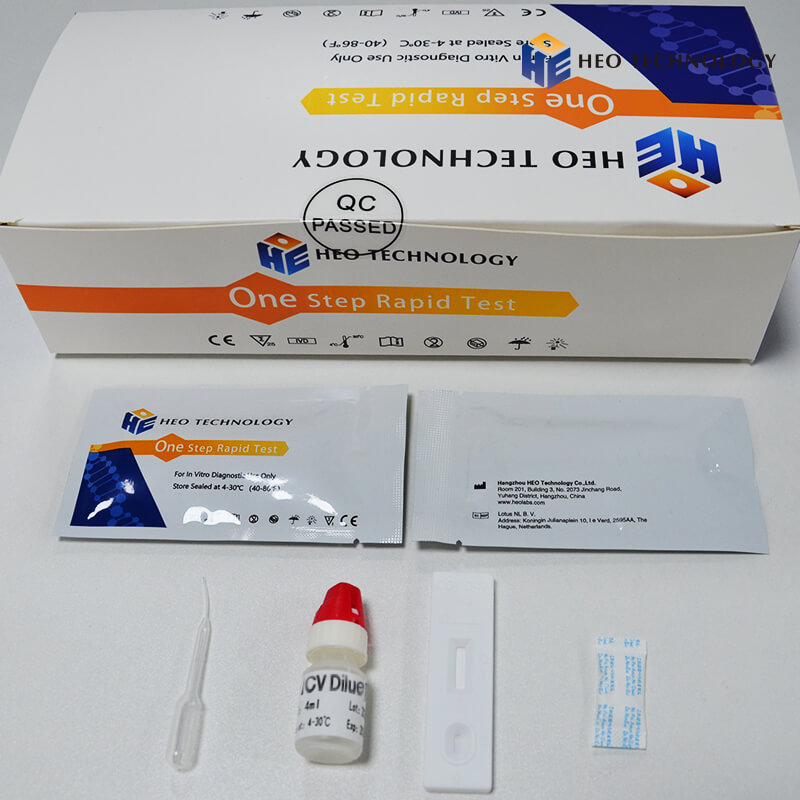
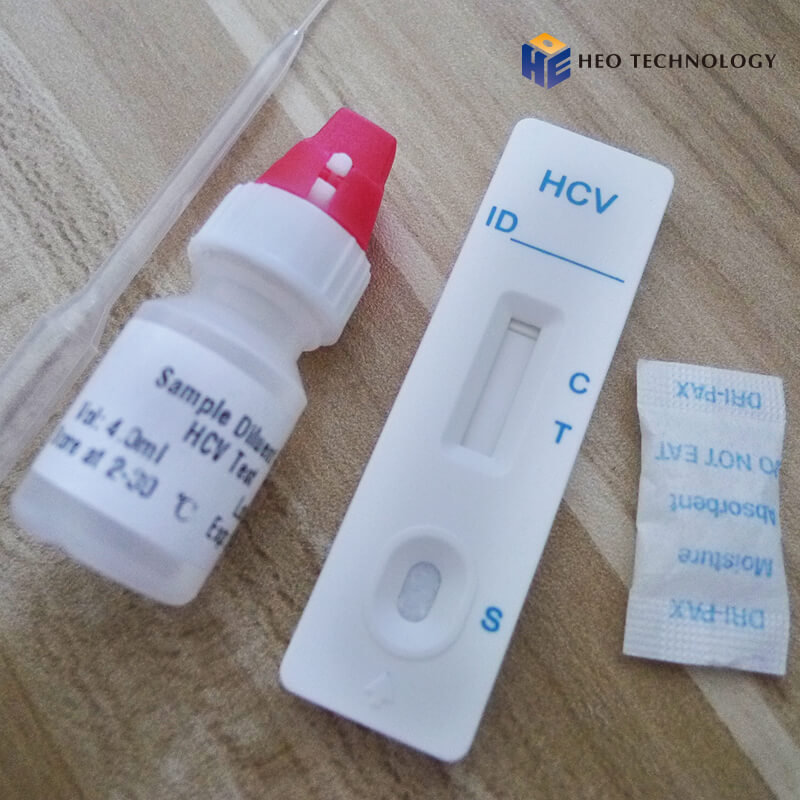
REAGENTS AND MATERIALS SUPPLIED
Test device individually foil pouched with a desiccant
• Plastic dropper.
• Sample Diluent
• package Insert
MATERIALS REQUIRED BUT NOT PROVIDED
Positive and negative controls (available as a separate item)
STORAGE & STABILITY
The test kits must be stored at 2-30℃in the sealed pouch and under dry conditions.
WARNINGS AND PRECAUTIONS
1) All positive results must be confirmed by an alternative method.
2) Treat all specimens as though potentially infectious. Wear gloves and protective clothing when handling specimens.
3) Devices used for testing should be autoclaved before disposal.
4) Do not use kit materials beyond their expiration dates.
5) Do not interchange reagents from different lots.
SAMPLE COLLECTION AND STORAGE
1) Collect Whole Blood /Serum / Plasma specimens following regular clinical laboratory procedures.
2) Storage: Whole Blood can not be frozen. A specimen should be refrigerated if not used the same day of collection. Specimens should be frozen if not used within 3 days of collecting. Avoid freezing and thawing the specimens more than 2-3 times before using. 0.1% of Sodium Azide can be added to specimen as a preservative without affecting the results of the assay.
ASSAY PROCEDURE
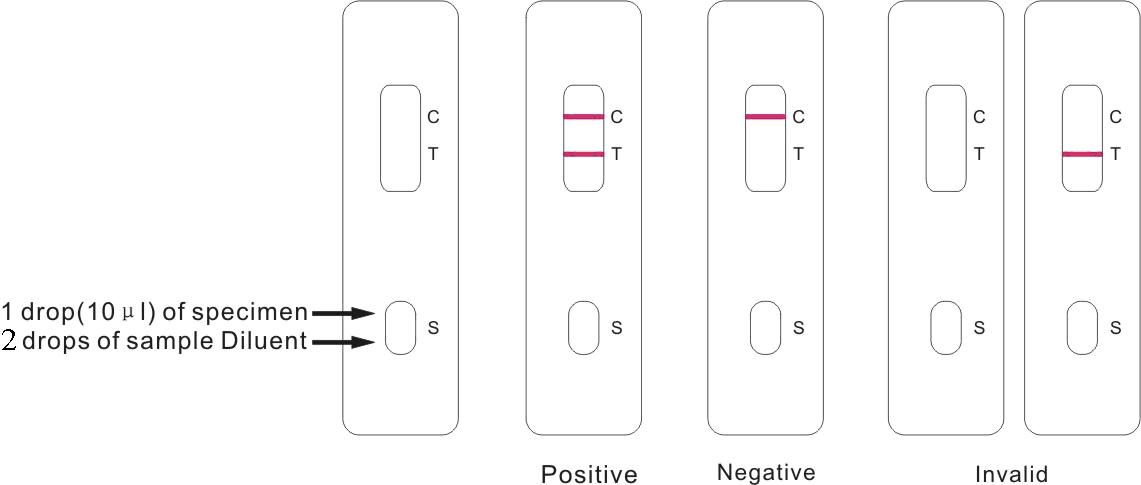
1) Using the enclosed plastic dropper for the sample, dispense 1 drop (10μl) of Whole Blood /Serum / Plasma to the circular sample well of the test card
2) Add 2 drops of Sample Diluent to the sample well, immediately after the specimen is added, from the dropper tip diluent vial (or all contents from the single test ampule).
3) Interpret test results at 15 minutes.
Notes:
1) Applying a sufficient amount of sample diluent is essential for a valid test result. If migration (the wetting of membrane) is not observed in the test window after one minute, add one more drop of diluent to the sample well.
2) The positive results could appear as soon as one minute for a sample with high levels of HCV antibodies.
3) Do Not Interpret results after 20 minutes
READING THE TEST RESULTS
1) Positive: Both a purplish red test band and a purplish red control band appear on the membrane. The lower the antibody concentration, the weaker the test band.
2) Negative: Only the purplish red control band appears on the membrane. The absence of a test band indicates a negative result.
3) Invalid result: There should always be a purplish red control band in the control region, regardless of the test result. If a control band is not seen, the test is considered invalid. Repeat the test using a new test device.
Note: It is normal to have a slightly lightened control band with very strong positive samples, as long as it is distinctly visible.
LIMITATION
1) Only clear, fresh, free flowing Whole Blood /Serum / Plasma can be used in this test.
2) Fresh samples are best but frozen samples can be used. If a sample has been frozen, it should be allowed to thaw in a vertical position and checked for fluidity. Whole Blood can not be frozen.
3) Do not agitate the sample. Insert a pipette just below the surface of the sample to collect the Specimen.
Product detail pictures:


Related Product Guide:
We believe in: Innovation is our soul and spirit. Quality is our life. Customer need is our God for Hot sale Factory Dengue Ns1 Test Device - ONE STEP HCV TEST (Whole Blood/Serum/Plasma) – HEO , The product will supply to all over the world, such as: Sao Paulo, South Korea, Georgia, We've got constructed strong and long co-operation relationship with an enormous quantity of companies within this business in Kenya and overseas. Immediate and professional after-sale service supplied by our consultant group has happy our buyers. Thorough Info and parameters from the merchandise will probably be sent for you for any thorough acknowledge. Free samples may be delivered and company check out to our corporation. n Kenya for negotiation is constantly welcome. Hope to get inquiries type you and construct a long-term co-operation partnership.
A nice supplier in this industry, after a detail and careful discussion, we reached a consensus agreement. Hope that we cooperate smoothly.