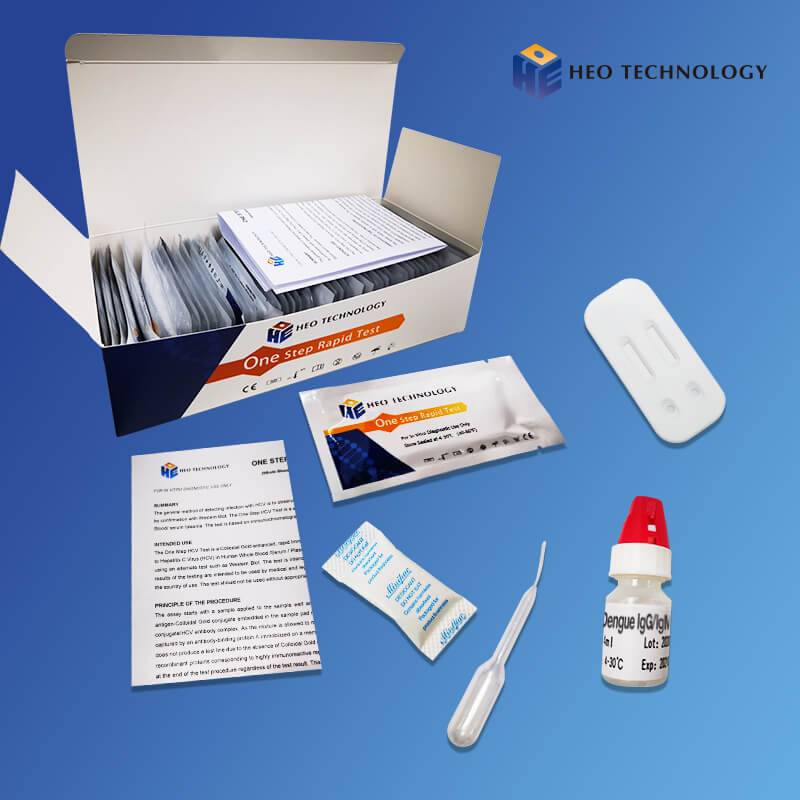
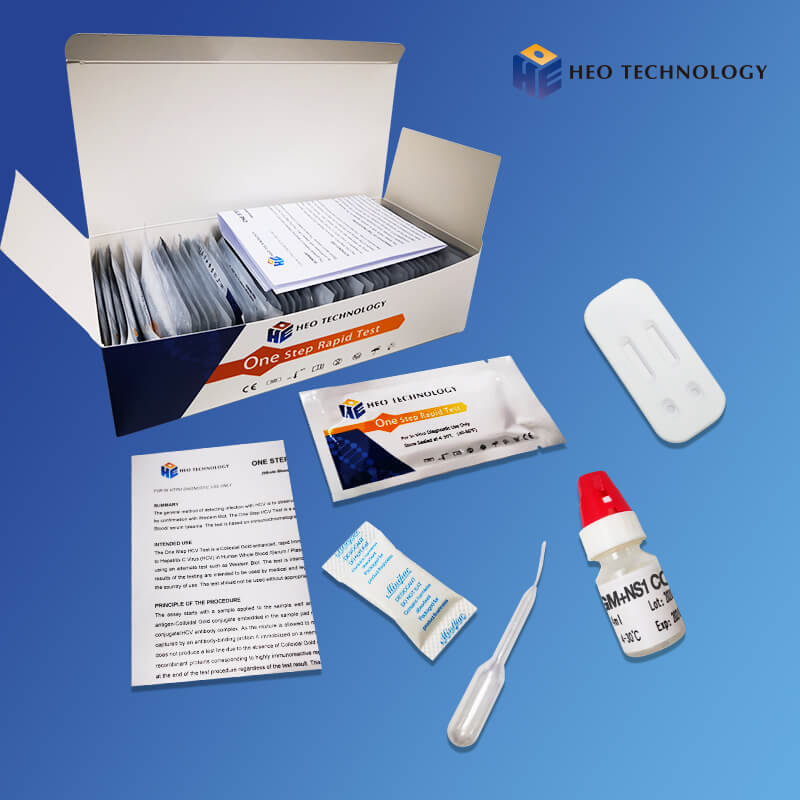
Wholesale Besure Rapid Diagnostic Test - Dengue Ns1 Test Device (Whole BloodSerumPlasma) – HEO
Wholesale Besure Rapid Diagnostic Test - Dengue Ns1 Test Device (Whole BloodSerumPlasma) – HEO Detail:
Dengue Ns1 Test Device (Whole BloodSerumPlasma)
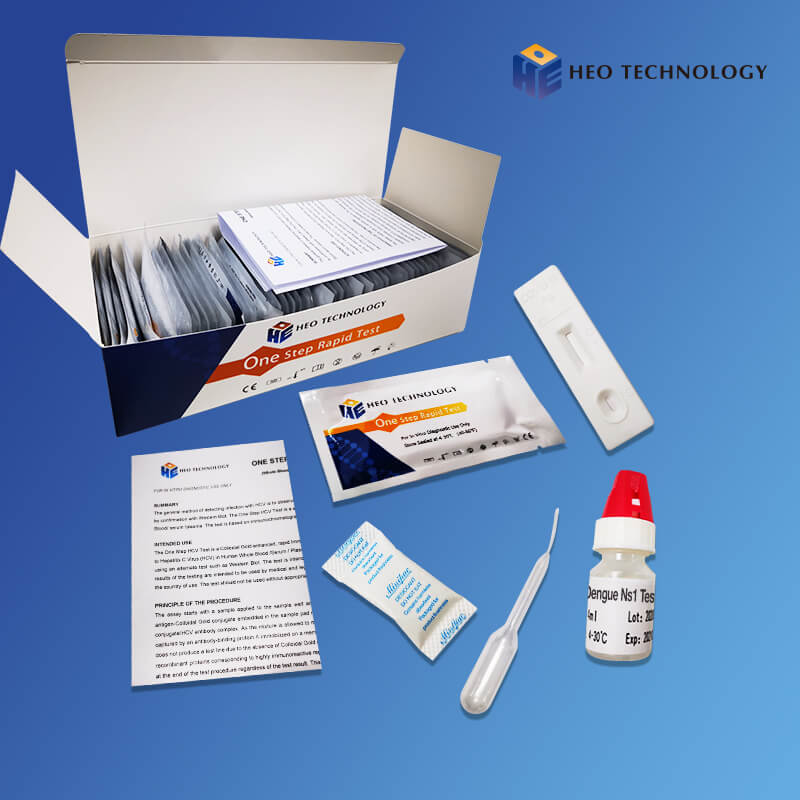



[INTENDED USE]
The Dengue NS1 Antigen Rapid Test Cassette/Strip is a lateral flow chromatographic immunoassay for the qualitative detection of antigens to Dengue viruses in human Whole Blood/Serum/Plasma. It provides an aid in the diagnosis of infection with Dengue viruses.
[SUMMARY]
Dengue fever is an acute vector-borne infectious disease caused by dengue virus transmitted by mosquitoes. Dengue virus infection can lead to recessive infection, dengue fever, dengue hemorrhagic fever, dengue hemorrhagic fever. Typical clinical manifestations of dengue fever include sudden onset, high fever, headache, severe muscle, bone and joint pain, skin rash, bleeding tendency, lymph node enlargement, decreased white blood cell count, thrombocytopenia and so on in some patients. This disease basically is in tropical and subtropical area popularity, because this disease is transmitted by Aides mosquito, reason popularity has certain seasonally, be in every year commonly in May ~ November, peak is in July ~ September. In the new epidemic area, the population is generally susceptible, but the incidence is mainly adult, in the endemic area, the incidence is mainly children.
[PRINCIPLE]
The Dengue NS1 Antigen Rapid Test Cassette/Strip is an immunoassay based on the principle of the double antibody-sandwich technique. During testing, anti-Dengue antibody is immobilized in the test line region of the device. After a Whole Blood/Serum/Plasma specimen is placed in the specimen well, it reacts with anti-Dengue antibody coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized anti-Dengue antibody. If the specimen contains dengue virus antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain dengue virus antigen, a colored line will not appear in this region indicating a negative result. To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
[STORAGE AND STABILITY]
Store as packaged in the sealed pouch at temperature (4-30℃ or 40-86℉). The kit is stable within the expiration date printed on the labeling.
Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
The LOT and the expiration date were printed on the labeling.
[SPECIMEN]
The test can be used to test Whole Blood/Serum/Plasma specimens.
Collect blood specimen (containing EDTA, citrate or heparin) by vein puncture following standard laboratory procedures.
Separate serum or plasma from blood as soon as possible to avoid hemolytic. Use only clear non-demolished specimens.
Store specimens at 2-8℃ (36-46℉) if not tested immediately. Store specimens at 2-8℃ up to 7 days. The specimens should be frozen at -20℃ (-4℉) for longer storage. Do not freeze whole blood specimens.
Avoid multiple freeze-thaw cycles. Prior to testing, bring frozen specimens to room temperature slowly and mix gently. Specimens containing visible particulate matter should be clarified by centrifugation before testing.
Do not use samples demonstrating gross lineman, gross hemolytic or turbidity in order to avoid interference on result interpretation.
[TEST PROCEDURE]
- Allow the test device and specimens to equilibrate to temperature (15-30℃or 59-86℉) prior to testing.
- [For Strip]
1. Remove the test strip from the sealed pouch and use it as soon as possible.
2. Place the test strip on a clean and level surface.
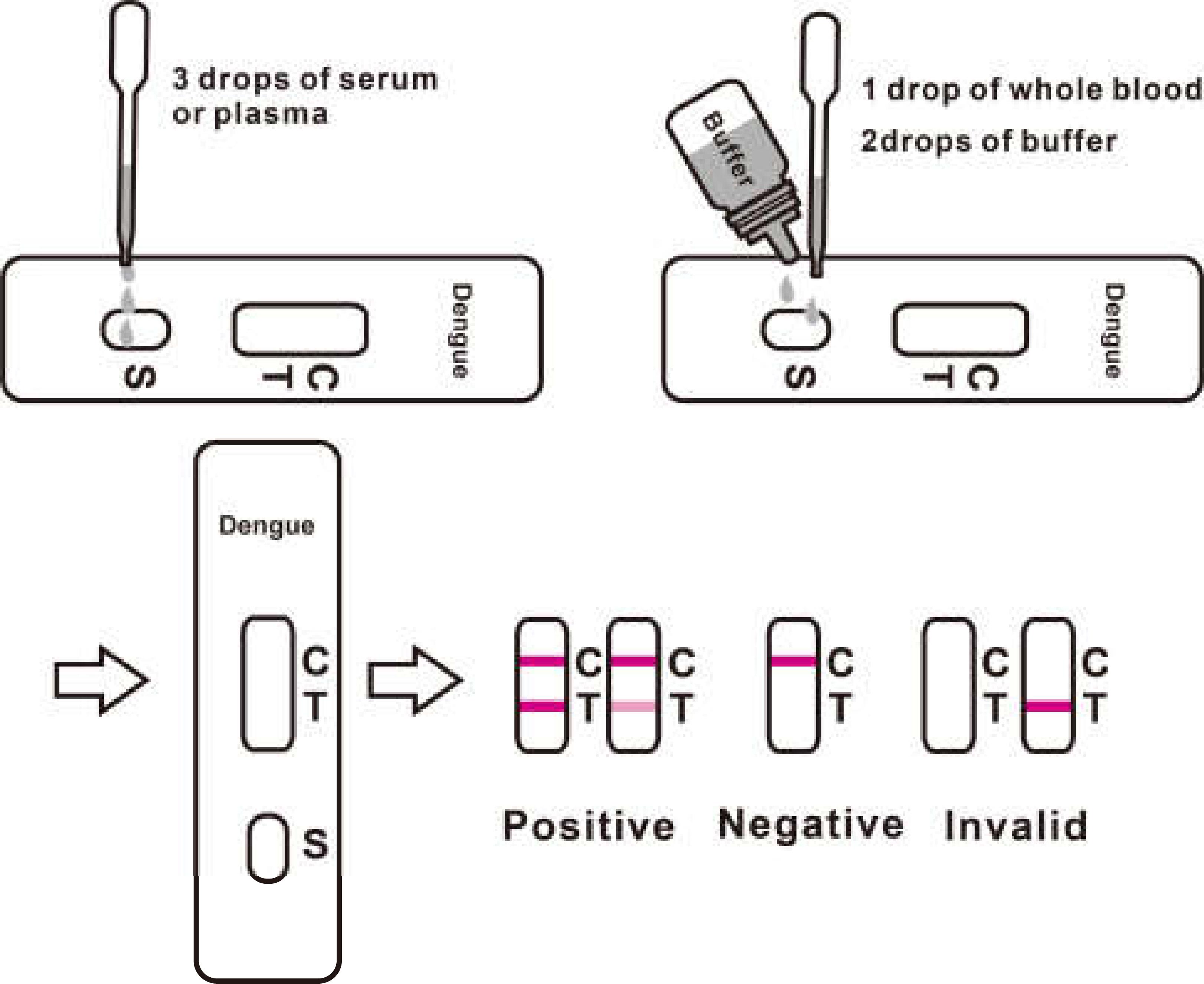
3. For serum or plasma specimen: Hold the dropper vertically and transfer 3 drops of serum or plasma (approximately 100μl) to the specimen pad of the test strip, then start the timer. See illustration below.
4. for whole blood specimens: Hold the dropper vertically and transfer 1 drop of whole blood (approximately 35μl) to the specimen pad of the test strip, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
5. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.

1.Remove the test cassette from the sealed pouch and use it as soon as Possible.
2.Place the test cassette on a clean and level surface.
3.For serum or plasma specimen: Hold the dropper vertically and transfer 3 drops of serum or plasma (approximately 100μl) to the specimen well (S) of the test cassette, then start the timer. See illustration below.
4.For whole blood specimens: Hold the dropper vertically and transfer 1 drop of whole blood(approximately 35μl) to the specimen well(S) of the test cassette, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
5.Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
[INTERPRETATION OF RESULTS]
Positive:*Two lines appear. One colored line should be in the control region (C), and another apparent colored line adjacent should be in the test region (T). This positive result indicates the presence of antigens to Dengue.
Negative: One colored line appears in the control region (C). No line appears in the test region (T). This negative result indicates the absence of antigens to Dengue.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test cassette/strip. If the problem persists, discontinue using the lot immediately and contact your local distributor.
Product detail pictures:
Related Product Guide:
Sticking for the perception of "Creating goods of high quality and making good friends with people today from all around the world", we constantly set the interest of shoppers to begin with for Wholesale Besure Rapid Diagnostic Test - Dengue Ns1 Test Device (Whole BloodSerumPlasma) – HEO , The product will supply to all over the world, such as: Algeria, Durban, America, Now we have a excellent team supplying specialist service, prompt reply, timely delivery, excellent quality and best price to our customers. Satisfaction and good credit to every customer is our priority. We have been sincerely looking forward to cooperate with customers all over the world. We believe we can satisfy with you. We also warmly welcome customers to visit our company and purchase our solutions.
In our cooperated wholesalers, this company has the best quality and reasonable price, they are our first choice.