Hot-selling Rapid Test Human Coronavirus - COVID-19 IgG/IgM Rapid Test Cassette (Colloidal gold) – HEO
Hot-selling Rapid Test Human Coronavirus - COVID-19 IgG/IgM Rapid Test Cassette (Colloidal gold) – HEO Detail:

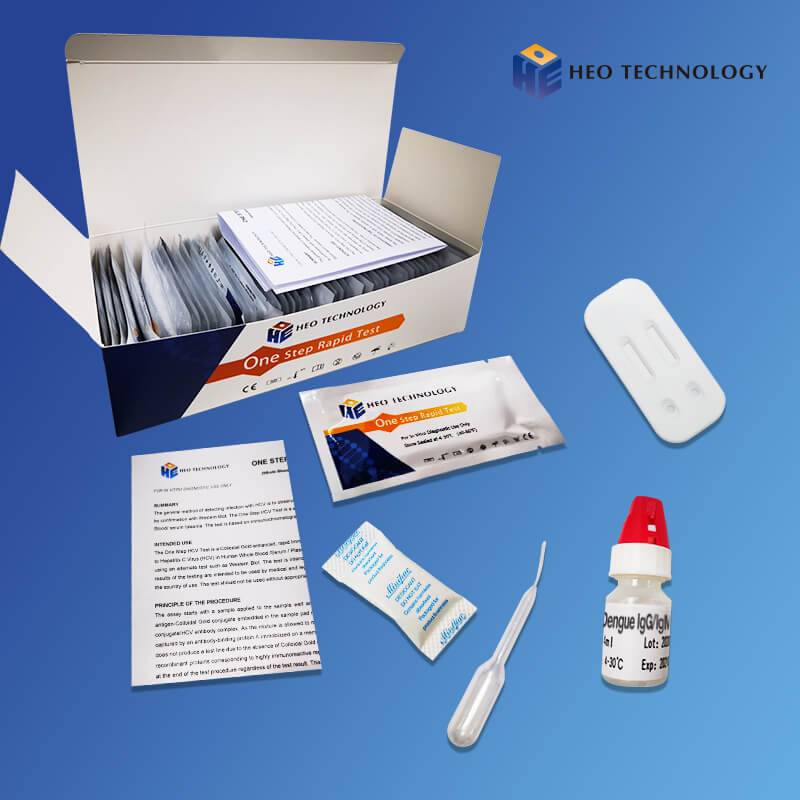
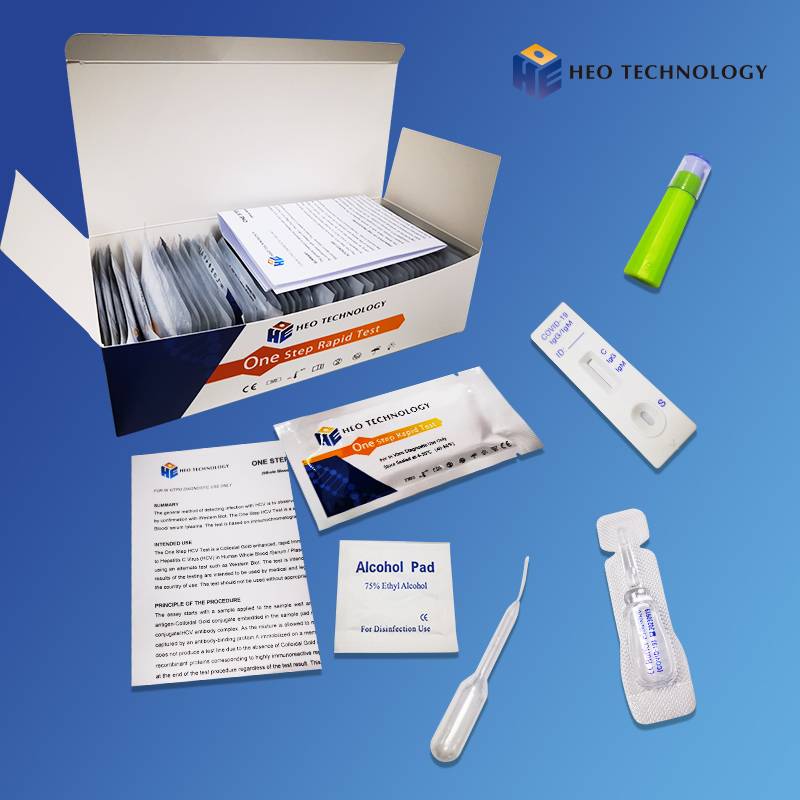
COVID-19 IgG/IgM Rapid Test Cassette (Colloidal gold)
Accurate, effective, commonly used.



1. [1INTENDED USE]
The COVID-19 Antigen Rapid Test Cassette is a lateral flow immunoassay intended for the qualitative detection SARS-CoV-2 nucleocapsid antigens in nasopharyngeal swab and oropharyngeal swab from individuals who are suspected of COVID-19 by their healthcare provider.
2. [STORAGE AND STABILITY]
Store as packaged in the sealed pouch at temperature (4-30℃ or 40-86℉). The kit is stable within the expiration date printed on the labeling.
Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
he LOT and the expiration date were printed on the labeling.
3. Sample Collection
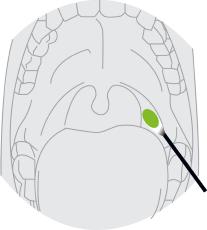
Nasopharyngeal Swab Sample
Insert minitip swab with a flexible shaft (wire or plastic) through the nostril parallel to the palate (not upwards) until resistance is encountered or the distance is equivalent to that from the ear to the nostril of the patient, indicating contact with the nasopharynx. Swab should reach depth equal to distance from nostrils to outer opening of the ear. Gently rub and roll the swab. Leave swab in place for several seconds to absorb secretions. Slowly remove swab while rotating it. Specimens can be collected from both sides using the same swab, but it is not necessary to collect specimens from both sides if the minitip is saturated with fluid from the first collection. If a deviated septum or blockage creates difficulty in obtaining the specimen from one nostril, use the same swab to obtain the specimen from the other nostril.
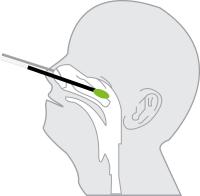
Oropharyngeal Swab Sample
Insert swab into the posterior pharynx and tonsillar areas. Rub swab over both tonsillar pillars and posterior oropharynx and avoid touching the tongue, teeth, and gums.
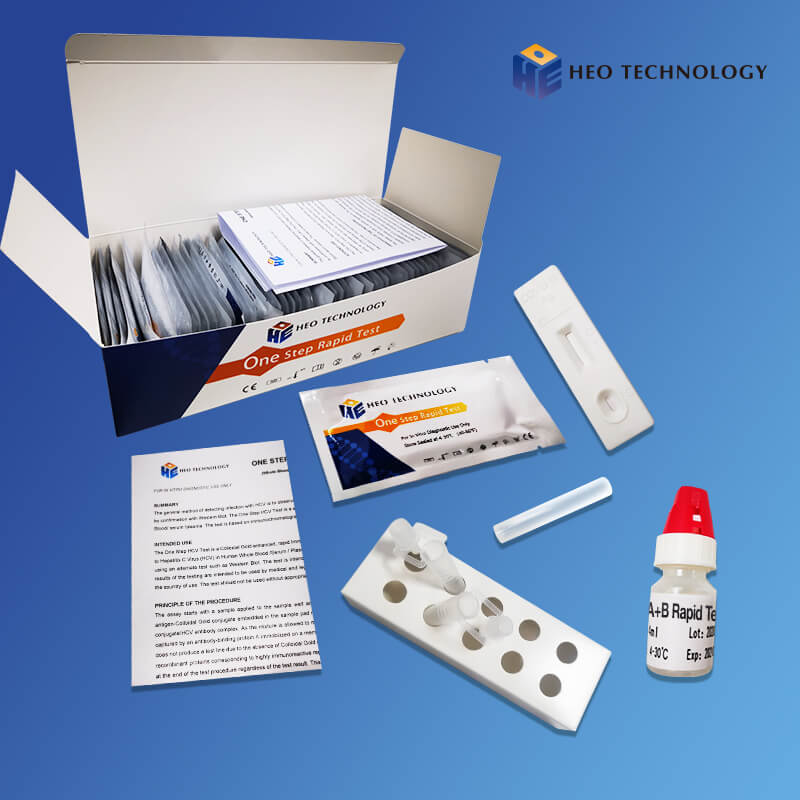
Sample Preparation
After Swab specimens were collected, swab can be stored in extraction reagent provided with the kit. Also can be stored by immersing the swab head in a tube containing 2 to 3 mL of virus preservation solution (or isotonic saline solution, tissue culture solution, or phosphate buffer).
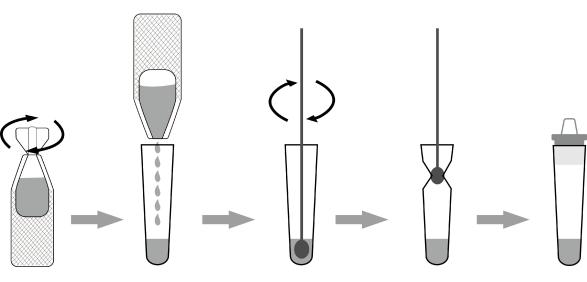
[SPECIMEN PREPARATION]
1. Unscrew the lid of an extraction reagent. Add all of the specimen extraction reagent into an extraction tube, and put it on the work station.
2. Insert the swab sample into the extraction tube which contains extraction reagent. Roll the swab at least 5 times while pressing the head against the bottom and side of the extraction tube. Leave the swab in the extraction tube for one minute.
3. Remove the swab while squeezing the sides of the tube to extract the liquid from the swab. The extracted solution will be used as test specimen.
4. Insert a dropper tip into the extraction tube tightly.
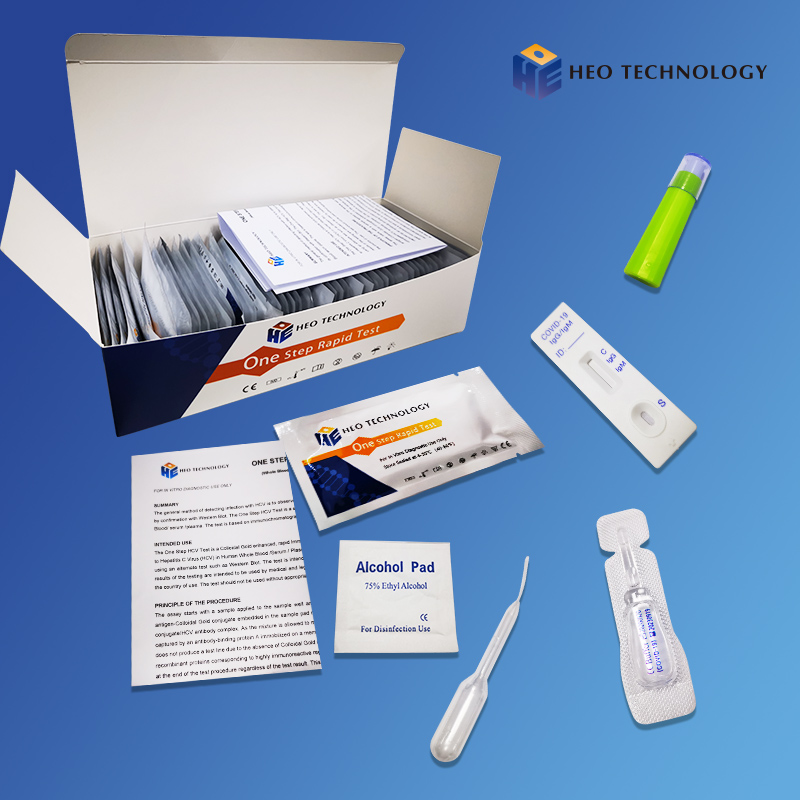
[TEST PROCEDURE]
1. Allow the test device and specimens to equilibrate to temperature (15-30℃ or 59-86℉) prior to testing.
2. Remove the test cassette from the sealed pouch.
3. Reverse the specimen extraction tube, holding the specimen extraction tube upright, transfer 3 drops (approximately 100μL) to the specimen well(S) of the test cassette, then start the timer. See illustration below.
4. Wait for colored lines to appear. Interpret the test results at 15 minutes. Do not read results after 20 minutes.
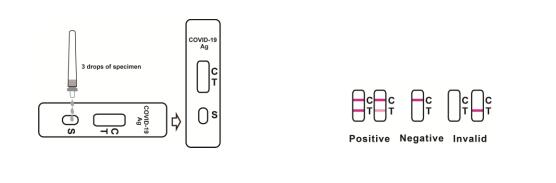
[INTERPRETATION OF RESULTS]
Positive:*Two lines appear. One colored line should be in the control region (C), and another apparent colored line adjacent should be in the test region (T). Positive for the presence of SARS-CoV-2 nucleocapsid antigen. Positive results indicate the presence of viral antigens but clinical correlation with patient history and other diagnostic information is necessary to determine infection status Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative: One colored line appears in the control region (C). No line appears in the test region (T). Negative results are presumptive. Negative test results do not preclude infection and should not be used as the sole basis for treatment or other patient management decisions, including infection control decisions, particularly in the presence of clinical signs and symptoms consistent with COVID-19, or in those who have been in contact with the virus. It is recommended that these results be confirmed by a molecular testing method, if necessary, for patient management.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test using a new test cassette. If the problem persists, discontinue using the lot immediately and contact your local distributor.
Product detail pictures:


Related Product Guide:
We aim to find out quality disfigurement from the production and supply the best service to domestic and overseas customers wholeheartedly for Hot-selling Rapid Test Human Coronavirus - COVID-19 IgG/IgM Rapid Test Cassette (Colloidal gold) – HEO , The product will supply to all over the world, such as: Buenos Aires, Barbados, Milan, Why we can do these? Because: A, We are honest and reliable. Our items have high quality, attractive price, sufficient supply capacity and perfect service. B, Our geographical position has a big advantage . C, Various types: Welcome your inquiry, It might be highly appreciated.
It's really lucky to find such a professional and responsible manufacturer, the product quality is good and delivery is timely, very nice.