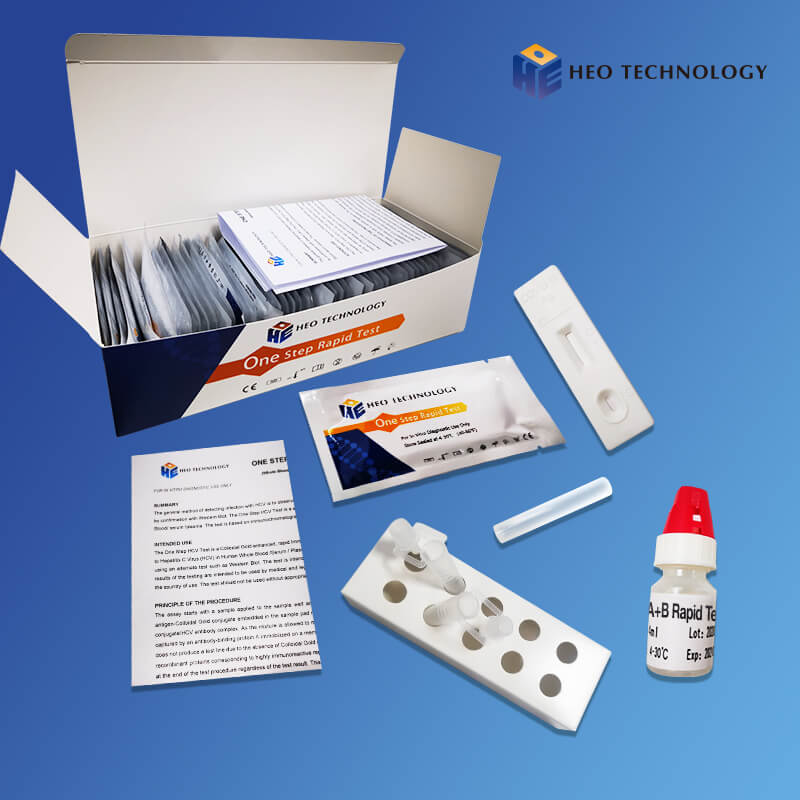
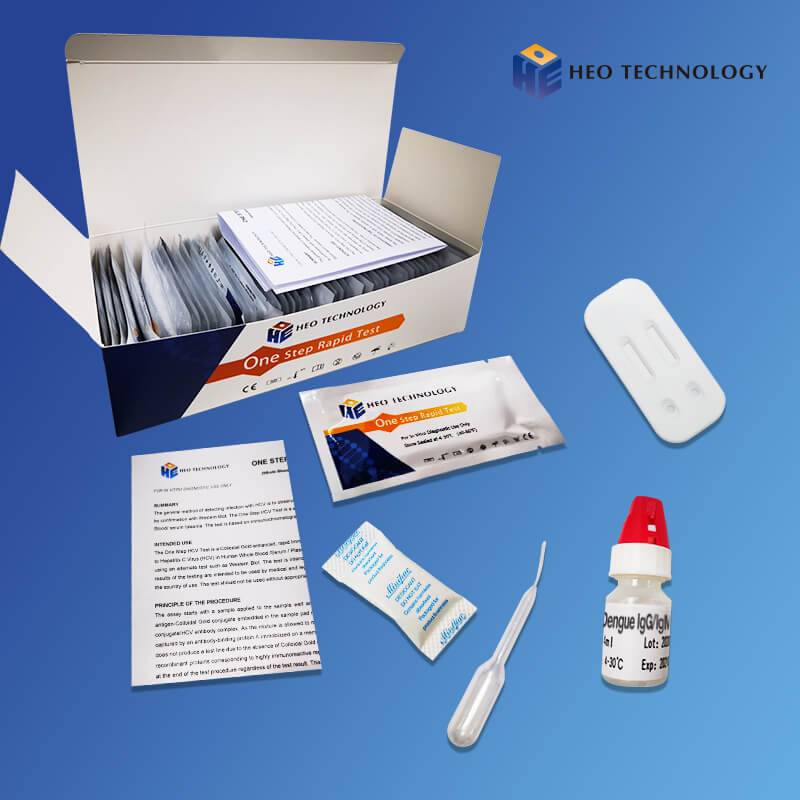
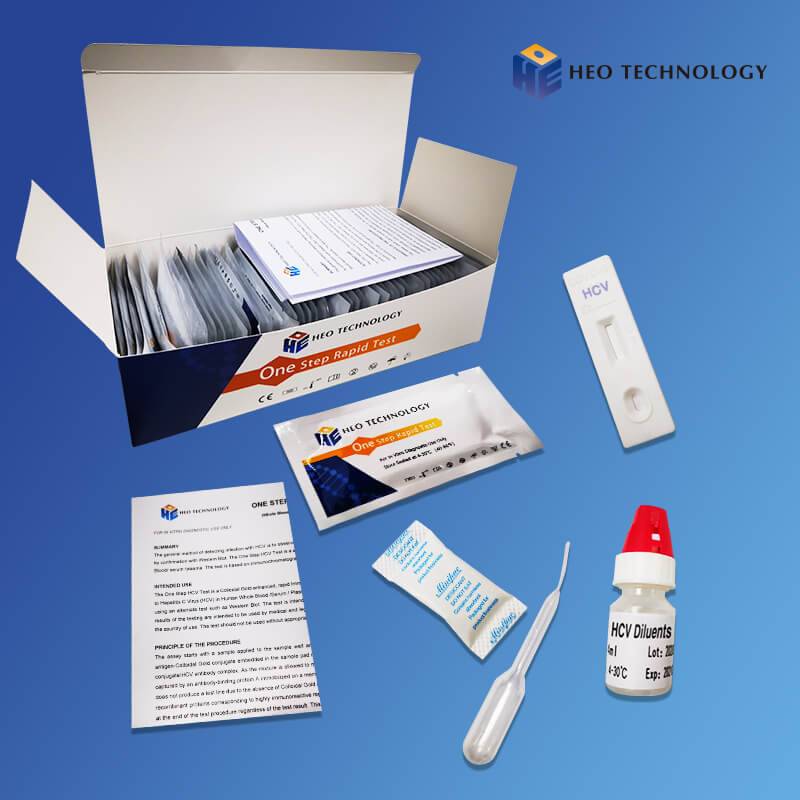
Hot Sale for Iga Igm Ige Igg - Dengue IgGIgM+Ns1 Combo Test Device (Whole BloodSerumPlasma) – HEO
Hot Sale for Iga Igm Ige Igg - Dengue IgGIgM+Ns1 Combo Test Device (Whole BloodSerumPlasma) – HEO Detail:
Dengue IgGIgM+Ns1 Combo Test Device (Whole BloodSerumPlasma)
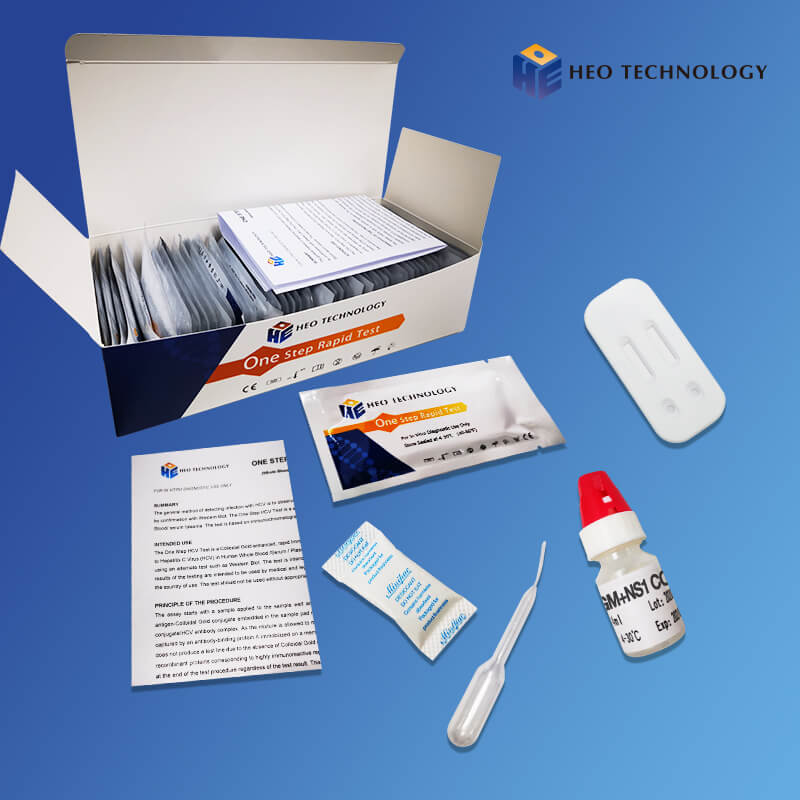
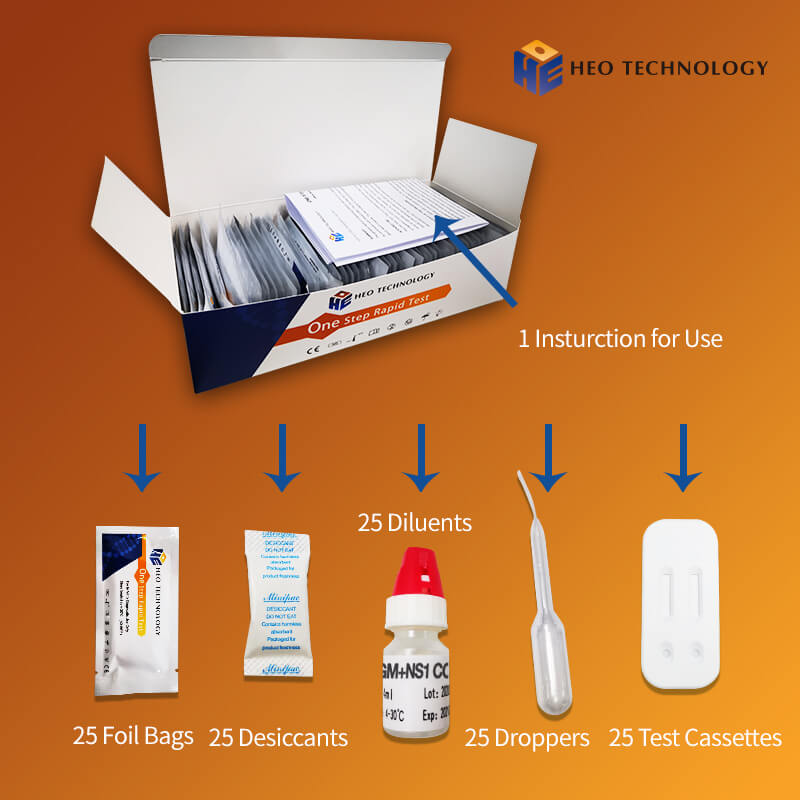
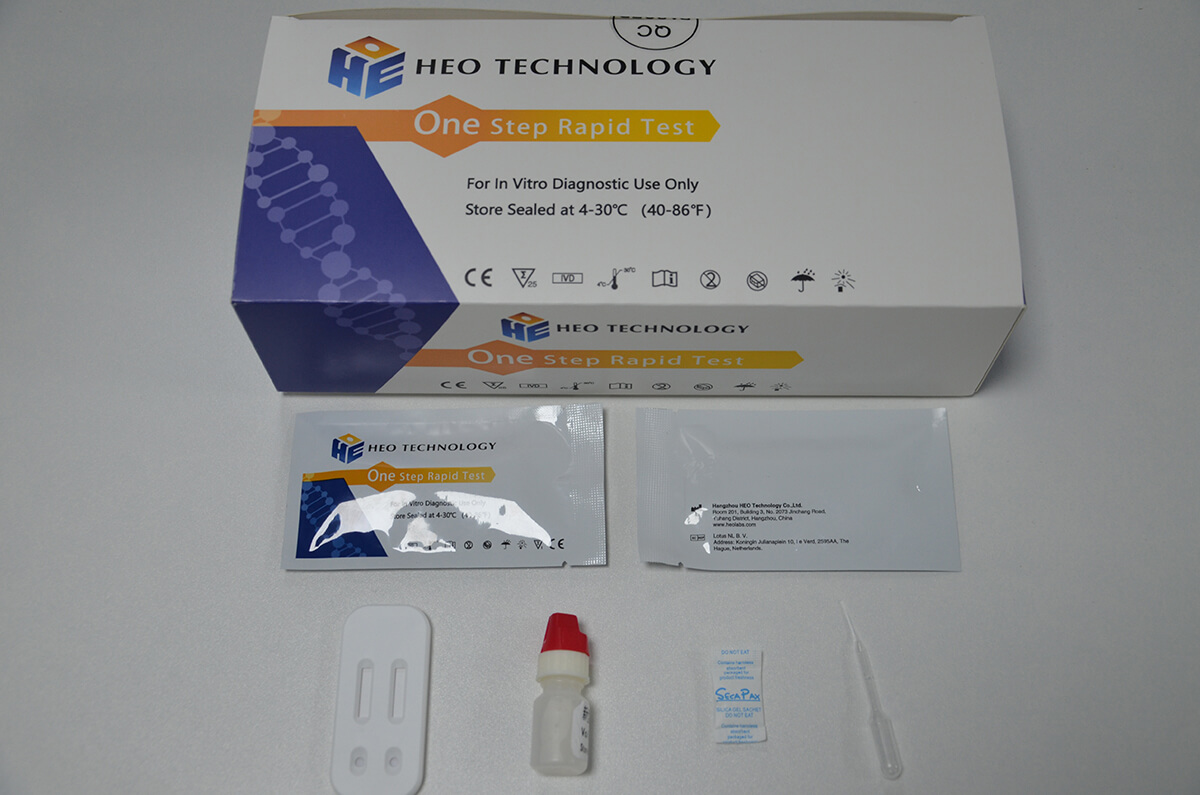
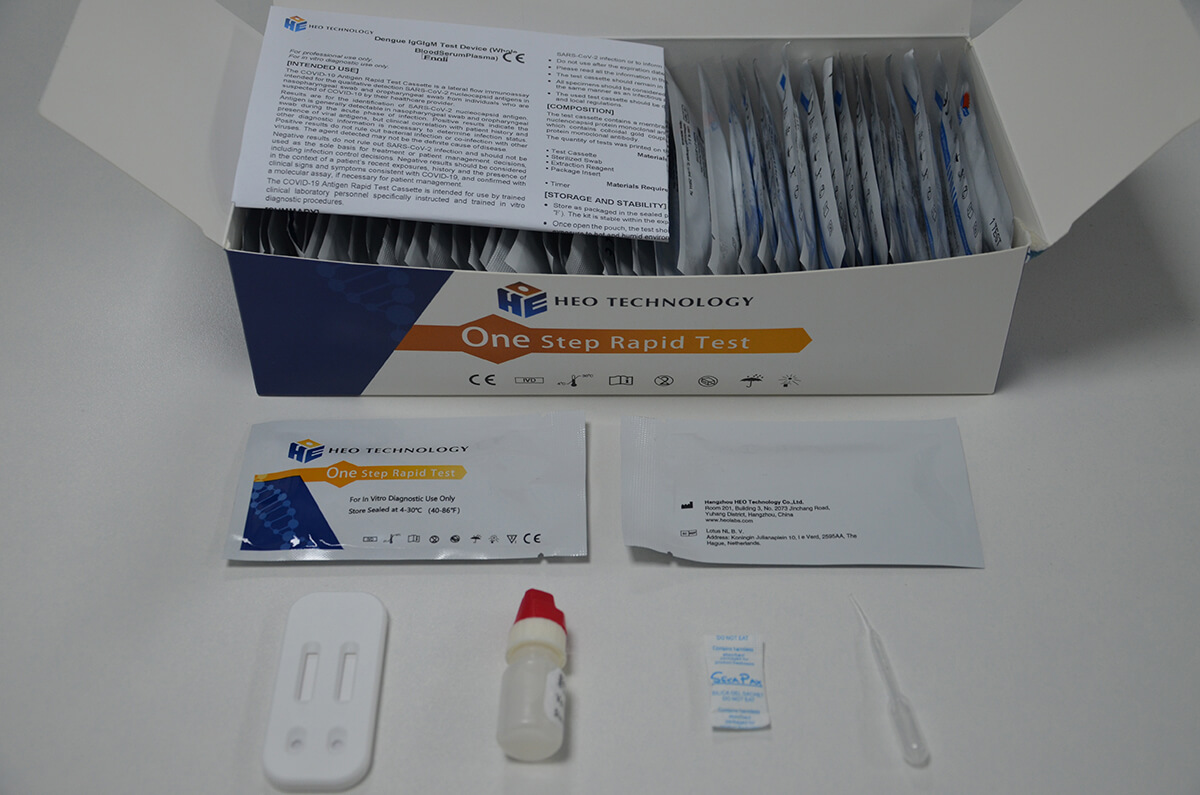
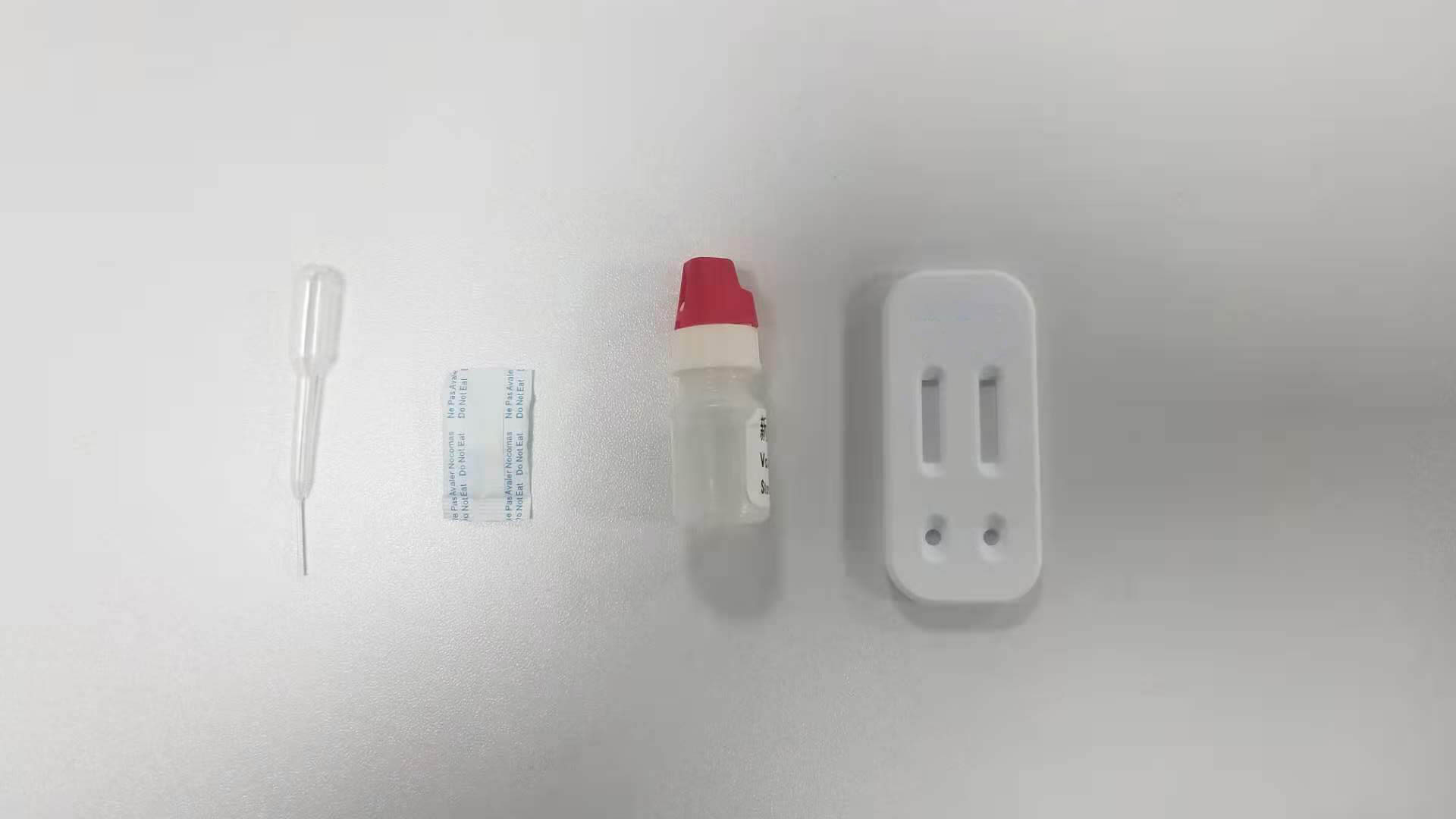

Intended Use
The Dengue NS1 Ag-IgG/IgM Combo Test is a rapid chromatographic immunoassay for the qualitative detection of antibodies (IgG and IgM) and dengue virus NS1 antigen to dengue virus in Serum or Plasma to aid in the diagnosis of Dengue viral infection.
[SUMMARY]
Dengue fever is an acute vector-borne infectious disease caused by dengue virus transmitted by mosquitoes. Dengue virus infection can lead to recessive infection, dengue fever, dengue hemorrhagic fever, dengue hemorrhagic fever. Typical clinical manifestations of dengue fever include sudden onset, high fever, headache, severe muscle, bone and joint pain, skin rash, bleeding tendency, lymph node enlargement, decreased white blood cell count, thrombocytopenia and so on in some patients. This disease basically is in tropical and subtropical area popularity, because this disease is transmitted by Aides mosquito, reason popularity has certain seasonally, be in every year commonly in May ~ November, peak is in July ~ September. In the new epidemic area, the population is generally susceptible, but the incidence is mainly adult, in the endemic area, the incidence is mainly children.
Principle
The Dengue NS1 Ag-IgG/IgM Combo Test is a qualitative membrane strip based immunoassay for the detection of dengue virus antibodies (IgG and IgM) and dengue virus NS1 antigen in Serum or Plasma.
For IgG/IgM Test :The test device consists of: 1) a burgundy colored conjugate pad containing dengue recombinant envelope antigens conjugated with Colloid gold (dengue conjugates), 2) a nitrocellulose membrane strip containing two test lines (T1 and T2 lines) and a control line (C line). The T1 line is pre-coated with the antibody for the detection of IgM anti-dengue, T2 line is coated with antibody for the detection of IgG anti-dengue. When an adequate volume of test specimen is dispensed into the sample well of the test cassette, the specimen migrates by capillary action across the cassette. IgG anti-dengue, if present in the specimen, will bind to the dengue conjugates. The immunocomplex is then captured by the reagent pre-coated on the T2 band, forming a burgundy colored T2 line, indicating a dengue IgG positive test result and suggesting a recent or repeat infection. IgM anti-dengue if present in the specimen will bind to the dengue conjugates. The immunocomplex is then captured by the reagent coated on the T1 line, forming a burgundy colored T1 line, indicating a dengue IgM positive test result and suggesting a fresh infection. Absence of any T lines (T1 and T2) suggests a negative result.
For NS1 Test: In this test procedure, anti-Dengue NS1 antibody is immobilized in the test line region of the cassette. After a Whole Blood /Serum / Plasma specimen is placed in the specimen well, it reacts with anti-Dengue NS1 antibody coated particles that have been applied to the specimen pad. This mixture migrates chromatographically along the length of the test strip and interacts with the immobilized anti-Dengue NS1 antibody. If the specimen contains dengue virus NS1 antigen, a colored line will appear in the test line region indicating a positive result. If the specimen does not contain dengue virus NS1 antigen, a colored line will not appear in this region indicating a negative result.
To serve as a procedural control, a colored line will always appear at the control line region indicating that proper volume of specimen has been added and membrane wicking has occurred.
Storage and Stability
Store as packaged in the sealed pouch at room temperature or refrigerated (4-30℃ or 40-86℉). The test device is stable through the expiration date printed on the sealed pouch.
The test must remain in the sealed pouch until use.
Specimen Collection and Preparation
1. The Dengue NS1 Ag-IgG/IgM Combo Test can be performed used on Serum or Plasma.
2. To collect whole blood, serum or plasma specimens following regular clinical laboratory procedures.
3. Testing should be performed immediately after specimen collection. Do not leave the specimens at room temperature for prolonged periods. For long term storage, specimens should be kept below-20℃.
4. Bring specimens to room temperature prior to testing. Frozen specimens must be completely thawed and mixed well prior to testing. Specimens should not be frozen and thawed repeatedly.
Test Procedure
Allow the test, specimen, buffer and/or controls to reach room temperature 15-30℃ (59-86℉) prior to testing.
1. Bring the pouch to room temperature before opening it. Remove the test device from the sealed pouch and use it as soon as possible. Place the test device on a clean and level surface.
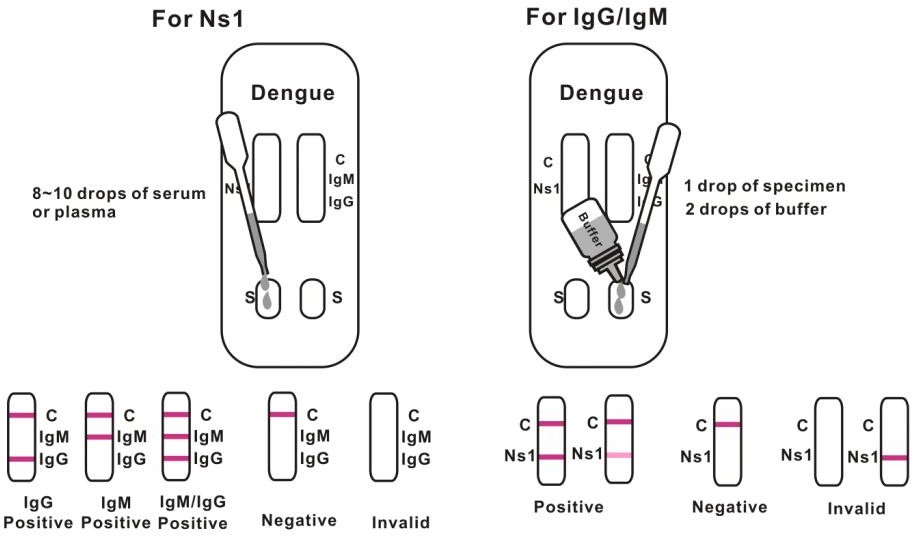
2. For IgG/IgM Test :Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
3. For NS1 Test: Hold the dropper vertically and transfer 8~10 drops of serum or plasma (approximately 100μl) to the specimen well(S) of the test device, then start the timer. See illustration below.
4. Wait for the colored line(s) to appear. Read results at 15 minutes. Do not interpret the result after 20 minutes.
Interpretation of Results
Positive:
For IgG/IgM Test: Control line and at least one test line appear on the membrane. The appearance of T2 test line indicates the presence of dengue specific IgG antibodies. The appearance of T1 test line indicates the presence of dengue specific IgM antibodies. And if both T1 and T2 line appear, it indicates that the presence of both dengue specific IgG and IgM antibodies. The lower the antibody concentration is, the weaker the result line is.
For NS1 Test: Two lines appear. One line should always appear in the control line region(C), and another one apparent colored line should appear in the test line region.
Negative:
One colored line appears in the control region(C).No apparent colored line appear in the test line region.
Invalid: Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test device. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
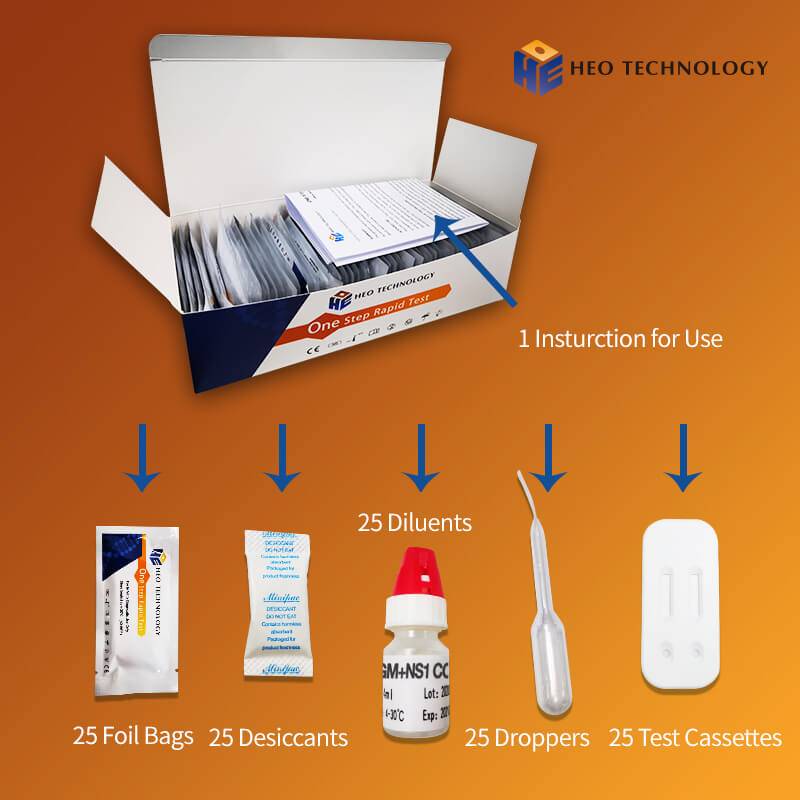
Product detail pictures:

Related Product Guide:
Being supported by an highly developed and skilled IT group, we could offer you technical support on pre-sales & after-sales support for Hot Sale for Iga Igm Ige Igg - Dengue IgGIgM+Ns1 Combo Test Device (Whole BloodSerumPlasma) – HEO , The product will supply to all over the world, such as: Nicaragua, Detroit, Kuala Lumpur, Our market share of our products and solutions has greatly increased yearly. If you are interested in any of our products or would like to discuss a custom order, make sure you feel free to contact us. We've been looking forward to forming successful business relationships with new clients around the world in the near future. We've been looking forward to your inquiry and order.
This is a honest and trustworthy company, technology and equipment are very advanced and the prodduct is very adequate, there is no worry in the suppliment.