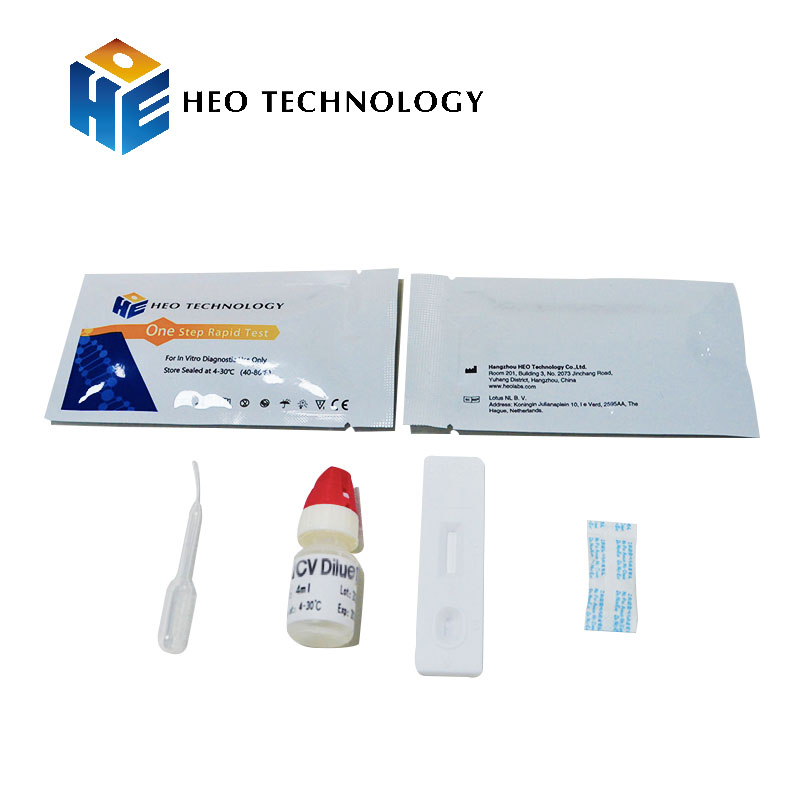
HCV Rapid Test cassette (WB/S/P)
HCV Rapid Test Cassette/Strip/kit (WB/S/P)




[INTENDED USE]
The HCV Rapid Test Cassette/Strip is a lateral flow chromatographic immunoassay for the qualitative detection of antibodies to Hepatitis C Virus in Whole Blood/Serum/Plasma. It provides an aid in the diagnosis of infection with Hepatitis C Virus.
[SUMMARY]
Hepatitis C virus (HCV) is a single stranded RNA virus of the Flaviviridae family and is the causative agent of Hepatitis C. Hepatitis C is a chronic disease affecting approximately 130-170 million people worldwide. According to the WHO, annually, more than 350,000 people die from hepatitis C-related liver diseases and 3-4 million people are infected with HCV. Approximately 3% of the world’s population is estimated to be infected with HCV. More than 80% of HCV-infected individuals develop chronic liver diseases, 20-30% develop cirrhosis after 20-30 yr, and 1-4% die from cirrhosis or liver cancer. Individuals infected with HCV produce antibodies to the virus and the presence of these antibodies in the blood indicates present or past infection with HCV.
[COMPOSITION] (25sets/ 40sets/50sets/customized specification are all approval)
The test cassette/strip contains a membrane strip coated with combination HCV antigen on the test line, rabbit antibody on the control line, and a dye pad which contains colloidal gold coupled with recombine HCV antigen. The quantity of tests was printed on the labeling.
Materials Provided
Test cassette/strip
Package insert
Buffer
Materials Required But Not Provided
Specimen collection container
Timer
Conventional methods fail to isolate the virus in cell culture or visualize it by electron microscope. Cloning the viral genome has made it possible to develop serologic assays that use recombinant antigens. Compared to the first generation HCV EIAs using single recombinant antigen, multiple antigens using recombinant protein and/or synthetic peptides have been added in new serologic tests to avoid nonspecific cross-reactivity and to increase the sensitivity of the HCV antibody tests. The HCV Rapid Test Cassette/Strip detects antibodies to HCV infection in Whole Blood/Serum/Plasma. The test utilizes a combination of protein A coated particles and recombinant HCV proteins to selectively detect antibodies to HCV. The recombinant HCV proteins used in the test are encoded by the genes for both structural (nucleocapsid) and non-structural proteins.
[PRINCIPLE]
The HCV Rapid Test Cassette/Strip is an immunoassay based on the principle of the double antigen-sandwich technique. During testing, a Whole Blood/Serum/Plasma specimen migrates upward by capillary action. The antibodies to HCV if present in the specimen will bind to the HCV conjugates. The immune complex is then captured on the membrane by the pre-coated recombinant HCV antigens, and a visible colored line will show up in the test line region indicating a positive result. If antibodies to HCV are not present or are present below the detectable level, a colored line will not form in the test line region indicating a negative result.
To serve as a procedural control, a colored line will always appear at the control line region, indicating that proper volume of specimen has been added and membrane wicking has occurred.
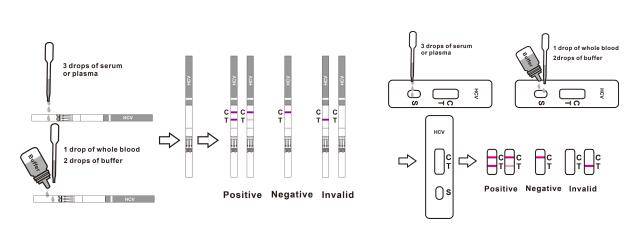
(The picture is for reference only, please refer to the material object.) [For Cassette]
Remove the test cassette from the sealed pouch.
For serum or plasma specimen: Hold the dropper vertically and transfer 3 drops of serum or plasma (approximately 100μl) to the specimen well (S) of the test device, then start the timer. See illustration below.
For whole blood specimens: Hold the dropper vertically and transfer 1 drop of whole blood(approximately 35μl) to the specimen well(S) of the test device, then add 2 drops of buffer (approximately 70μl) and start the timer. See illustration below.
Wait for colored line(s) to appear. Interpret the test results in 15 minutes. Do not read results after 20 minutes.
[WARNINGS AND PRECAUTIONS]
For in vitro diagnostic use only.
For healthcare professionals and professionals at point of care sites.
Do not use after the expiration date.
Please read all the information in this leaflet before performing the test.
The test cassette/strip should remain in the sealed pouch until use.
All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
The used test cassette/strip should be discarded according to federal, state and local regulations.
[QUALITY CONTROL]
A procedural control is included in the test. A colored line appearing in the control region (C) is considered an internal procedural control. It confirms sufficient specimen volume, adequate membrane wicking and correct procedural technique.
Control standards are not supplied with this kit. However, it is recommended that positive and negative controls be tested as good laboratory practice to confirm the test procedure and to verify proper test performance.
[LIMITATIONS]
The HCV Rapid Test Cassette/Strip is limited to provide a qualitative detection. The intensity of the test line does not necessarily correlate to the concentration of the antibody in the blood.
The results obtained from this test are intended to be an aid in diagnosis only. Each physician must interpret the results in conjunction with the patient’s history, physical findings, and other diagnostic procedures.
A negative test result indicates that antibodies to HCV are either not present or at levels undetectable by the test.
[PERFORMANCE CHARACTERISTICS]
Accuracy
Agreement with Commercial HCV Rapid Test
A side-by-side comparison was conducted using the HCV Rapid Test and commercially available HCV rapid tests. 1035 clinical specimens from three hospitals were evaluated with the HCV Rapid Test and the commercial kit. The specimens were checked with RIBA to confirm the presence of HCV antibody in the specimens. The following results are tabulated from these clinical studies:
| Commercial HCV Rapid Test | Total | |||
| Positive | Negative | |||
| HEO TECH® | Positive | 314 | 0 | 314 |
| Negative | 0 | 721 | 721 | |
| Total | 314 | 721 | 1035 | |
The agreement between these two devices is 100% for positive specimens, and 100% for negative specimens. This study demonstrated that the HCV Rapid Test is substantially equivalent to the commercial device.
Agreement with RIBA
300 clinical specimens were evaluated with the HCV Rapid Test and the HCV RIBA kit. The following results are tabulated from these clinical studies:
| RIBA | Total | |||
| Positive | Negative | |||
| HEO TECH® |
Positive |
98 | 0 | 98 |
|
Negative |
2 | 200 | 202 | |
| Total | 100 | 200 | 300 | |