COVID-19 Antigen Rapid Test Cassette (Colloidal Gold)
INTENDED USE
The COVID-19 Antigen Rapid Test Cassette (Colloidal Gold) is a lateral flow immunoassay intended for the qualitative detection SARS-CoV-2 nucleocap- sid antigens in nasal swab from individuals who are suspected of COVID-19 by their healthcare provider.
Results are for the identification of SARS-CoV-2 nucleocapsid antigen. Antigen is generally detectable in nasal swab during the acute phase of infection. Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status. Positive results do not rule out bacterial infection or co-infection with other viruses. The agent detected may not be the definite cause of disease.
Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions. Negative results should be considered in the context of a patient’s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19, and confirmed with a molecular assay, if necessary for patient management. This kit are for home use by laymen in a non-laboratory setting(such as person’s home or certain non-traditional sites such as offices,sporting events, schools etc.). The test results of this kit are for clinical reference only. It is recommended to conduct a comprehensive analysis of the condition based on the patients clinical manifestations and other laboratory tests.
SUMMARY
The novel coronaviruses (SARS-CoV-2) belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
PRINCIPLE
The COVID-19 Antigen Rapid Test Cassette (Nasal Swab) is a lateral flow immunoassay based on the principle of the double-antibody sandwich technique. SARS-CoV-2 nucleocapsid protein monoclonal antibody conjugat- ed with color microparticles is used as detector and sprayed on conjugation pad. During the test, SARS-CoV-2 antigen in the specimen interacts with SARS-CoV-2 antibody conjugated with color microparticles making antigen-antibody labeled complex. This complex migrates on the membrane via capillary action until the test line, where it will be captured by the pre-coat- ed SARS-CoV-2 nucleocapsid protein monoclonal antibody. A colored test line (T) would be visible in the result window if SARS-CoV-2 antigens are present in the specimen. Absence of the T line suggests a negative result. The control line (C) is used for procedural control, and should always appear if the test procedure is performed properly.
WARNINGS AND PRECAUTIONS
•For Self testing in vitro diagnostic use only.This tset cassette is for one-time use and cannot be reused or used by multiple people.
•Do not use this product as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status of COVID-19.
•Please read all the information in this leaflet before performing the test.
•Do not use this product after the expiration date.
•The test cassette should remain in the sealed pouch until use.
•All specimens should be considered potentially hazardous and handled in the same manner as an infectious agent.
•Test for children and young people should be used with an adult.
•The used test cassette should be discarded according to federal, state and local regulations.
•Do not use the test on children under 2 years old.
•Small children should be swabbed with the help of a second adult.
•Wash hands thoroughly before and after handling.
COMPOSITION
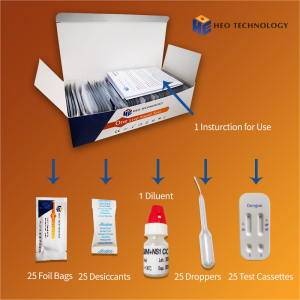
Materials Provided
•Test Cassettes: each cassette with desiccant in individual foil pouch
•Prepackaged Extraction Reagents:
•Sterilized Swabs: single use sterile swab for specimen collection
•Package Insert
Materials Required but not Provided
•Timer
STORAGE AND STABILITY
•Store as packaged in the sealed pouch at temperature (4-30℃ or 40-86℉). The kit is stable within the expiration date printed on the labeling.
•Once open the pouch, the test should be used within one hour. Prolonged exposure to hot and humid environment will cause product deterioration.
•DO NOT FREEZE.
SPECIMEN
Specimens obtained early during symptom onset will contain the highest viral titers; specimens obtained after five days of symptoms are more likely to produce negative results when compared to an RT-PCR assay. Inadequate specimen collection, improper specimen handling and/or transport may yield false results; therefore, training in specimen collection is highly recommended due to the importance of specimen quality to obtain accurate test results. Acceptable specimen type for testing is a direct nasal swab specimen obtained by the dual nares collection method. Prepare the extraction tube according to the Test Procedure and use the sterile swab provided in the kit for specimen collection.
Nasal Swab Specimen Collection
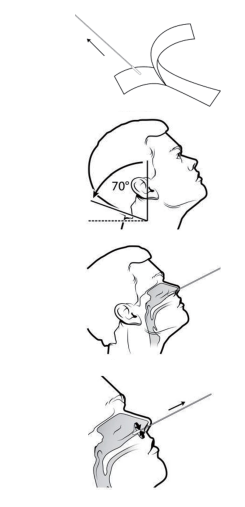
1.Remove the swab from the package.
2.Tilt patient's head back about 70°.
3.1-2While gently rotating the swab, insert swab about 2.5 cm (1 inch) into nostril until resistance is met at turbinates.
4.Rotate the swab several times against nasal wall and repeat in other nostril using the same swab.
Specimen Transport and Storage
Do not return the swab to the original swab packaging. Freshly collected specimens should be processed as soon as possible, but no later than one hour after specimen collection.
TEST PROCEDURE
Note: Allow the test cassettes, reagents and specimens to equilibrate to room temperature (15-30℃ or 59-86℉) prior to testing.
1.Place the extraction tube in the workstation.
2.Peel off aluminum foil seal from the top of the extraction tube containing the extraction tube containing the extraction buffer.
3.Sampling refers to section ‘Specimen Collection’.
4.Insert the nasal swab specimen into the extraction tube which contains extraction reagent. Roll the swab at least 5 times while pressing the head against the bottom and side of the extraction tube. Leave the nasal swab in the extraction tube for one minute.
5.Remove the nasal swab while squeezing the sides of the tube to extract the liquid from the swab. The extracted solution will be used as test sample. 6.Cover the extraction tube with a dropper tip tightly.
7.Remove the test cassette from the sealed pouch.
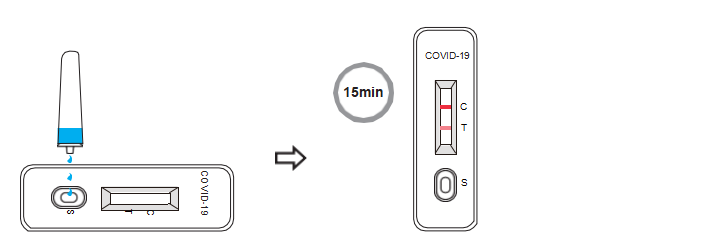
8.Reverse the specimen extraction tube, holding the tube upright, transfer 3 drops (approximately 100 μL) slowly to the specimen well (S) of the test cassette, then start the timer.
9.Wait for colored lines to appear. Interpret the test results at 15 minutes. Do not read results after 20 minutes.
[PERFORMANCE CHARACTERISTICS]
Clinical Performance
To estimate the clinical prerformance between the COVID-19 Antigen Rapid Test Cassette and the PCR comparotor, 628 nasal swab were collected from patients who were suspected of COVID-19.Summary data of COVID-19 Antigen Rapid Test Cassette (Nasal Swab) as below.
| COVID-19 antigen | RT-PCR | Total | ||
|
Positive |
Negative |
|||
|
HEO® |
Positive |
172 |
0 |
172 |
| Negative |
3 |
453 |
456 |
|
|
Total |
175 |
453 |
628 |
|
PPA =98.28% (172/175), (95%CI: 95.08%~99.64%) NPA =100% (453/453), (95%CI: 99.34%~100%)
PPA - Positive Percent Agreement (Sensitivity) NPA - Negative Percent Agreement (Specificity)
Limit of Detection (Analytical Sensitivity)
The study used cultured SARS-CoV-2 virus (Isolate USA-WA1/2020 NR- 52287), which is heat inactivated and spiked into nasal swab specimen. The Limit of Detection (LoD) is 1.0 ×102 TCID50/mL.
Cross Reactivity (Analytical Specificity)
Cross reactivity was evaluated by testing 32 commensal and pathogenic microorganisms that may be present in the nasal cavity.
No cross-reactivity was observed with recombinant MERS-CoV NP protein when tested at the concentration of 50 pg/mL.
No cross-reactivity was observed with the following viruses when tested at the concentration of 1.0×106 PFU/mL: Influenza A (H1N1), Influenza A (H1N1pdm09), Influenza A (H3N2), Influenza B (Yamagata), Influenza B (Victoria), Adenovirus (type 1, 2, 3, 5, 7, 55), Human metapneumovirus,
Parainfluenza virus (type 1, 2, 3, 4), Respiratory syncytial virus, Enterovirus, Rhinovirus, Human coronavirus 229E, Human coronavirus OC43, Human coronavirus NL63, Human coronavirus HKU1.
No cross-reactivity was observed with the following bacteria when tested at the concentration of 1.0×107 CFU/mL: Mycoplasma pneumoniae, Chlamy- dia pneumoniae, Legionella pneumophila, Haemophilus influenzae, Streptococcus pyogenes (group A), Streptococcus pneumoniae, Candida albicans,Staphylococcus aureus.
Interference
The following potential interference substances were evaluated with the COVID-19 Antigen Rapid Test Cassette (Nasal Swab) at the concentrations listed below and were found not to affect test performance.
| Substance Concentration | Substance Concentration |
| Mucin 2%
Benzocaine 5 mg/mL Saline nasal spray 15% Oxymetazoline 15% Tobramycin 5 μg/mL Oseltamivir phosphate 10 mg/mL Arbidol 5 mg/mL Fluticasone propionate 5% Triamcinolone 10 mg/mL |
Whole blood 4%
Menthol 10 mg/mL Phenylephrine 15% Mupirocin 10 mg/mL Zanamivir 5 mg/mL Ribavirin 5 mg/mL Dexamethasone 5 mg/mL Histamine 10 mg/mL dihydrochloride |
High-dose Hook Effect
The COVID-19 Antigen Rapid Test Cassette (Colloidal Gold) was tested up to 1.0×105 TCID50/mL of inactivated SARS-CoV-2 and no high-dose hook effect was observed.
Index of Symbol
Hangzhou HEO Technology Co., Ltd.
Adres:Room 201, Building 3, No. 2073 Jinchang Road, Yuhang District, Hangzhou,China
Postcode: 311113
Tel.: 0086-571-87352763 E-mail: 52558565@qq.com
Lotus NL B. V.
Address:Koningin Julianaplein 10, le Verd, 2595AA, The Hague, Netherlands.
E-mail: Peter@lotusnl.com Tel:+31644168999