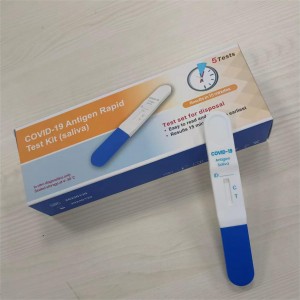
COVID-19 Antigen Rapid Test Kit (Saliva)
1.Store as packaged in the hermetically-sealed bag at the temperature (4-30℃
or 40-86℉) and avoid direct sunshine. The kit is stable within the expiration
date printed on the labeling.
2. Once the sealed bag is opened, the test should be used within one hour.
Prolonged exposure to hot and humid environments will cause product
deterioration.
3. The lot number and the expiration date are printed on each sealed bag.
2. If the fluid does not move upward , add 1 ml of drinking water to the
plastic bag with saliva, mix the water and saliva evenly , and then put the
absorbing pad back into the bag to absorb more saliva.
COVID -19 Antigen Rapid Test Kit (Saliva)
PACKING
1piece/box box or 5pieces/box or 25pieces/box
INTENDED USE
This product is suitable for the qualitative detection of novel coronavirus, or
COVID- 19, in Saliva. It aids in the diagnosis of infection with novel coronavirus.
SUMMARY
The novel coronaviruses (SARS-CoV-2) belong to the β genus. COVID-19 is an
acute respiratory infectious disease. People are generally susceptible to infection.
Currently, the patients infected by the novel coronavirus are the main source of
infection; asymptomatic infected people can also be an infectious source. Based on
the current epidemiological investigation, the incubation period is 1 to 14 days,
particularly 3 to 7 days. The main symptoms include fever, fatigue, and dry cough.
Nasal congestion, runny nose, sore throat, myalgia, and diarrhea are also found in
some cases.
PRINCIPLE
The COVID -19 Antigen Rapid Test Kit is an immunochromatographic membrane
assay that uses highly sensitive monoclonal antibodies to detect nucleocapsid
protein from SARS-CoV-2 in Saliva samples. The test strip is composed of the
following parts: namely sample pad, reagent pad, reaction membrane, and
absorbing pad. The reagent pad contains the colloidal-gold conjugated with the
monoclonal antibodies against the nucleocapsid protein of SARS-CoV-2; the
reaction membrane contains the secondary antibodies for nucleocapsid protein of
SARS-CoV-2. The whole strip is fixed inside a plastic device. When the sample is
added into the sample well, conjugates dried in the reagent pad are dissolved and
migrate along with the sample. If SARS-CoV-2 antigen presents in the sample, a
complex formed between the anti-SARS-2 conjugate and the virus will be captured
by the specific anti-SARS-2 monoclonal antibodies coated on the test line region
(T). Absence of the T line suggests a negative result. To serve as a procedural
control a red line will always appear in the control line region (C) indicating that
proper volume of sample has been added and membrane wicking has occurred.
COMPOSITION
1. Disposable test device
2. Disposable plastic saliva collection bag
Other device needed by not provided:
TEST PROCEDURE
Allow the test device and specimens to equilibrate to room temperature (15-
30℃ or 59-86℉) prior to testing.
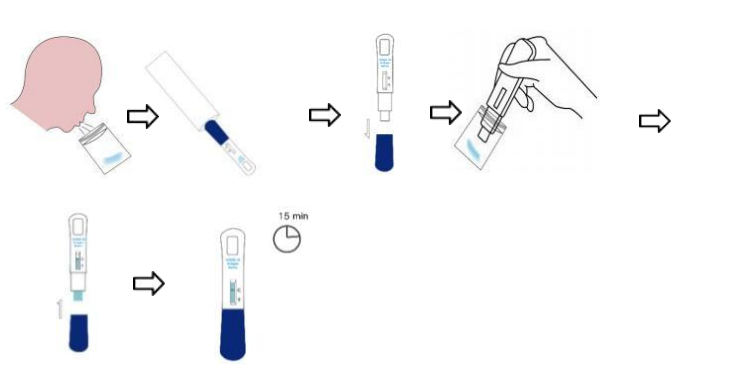
1.Collect at least 2 ml fresh saliva in a single use disposable plastic saliva
collection bag.
2. Open aluminum foil bag & take out the test cassette.
3. Take off the cassette cap.
4. Immerse the absorbing pad into the saliva bag and wait 2 minutes.
5. Remove the test card from saliva bag, then put back the cap and lay
down the test cassette on a flat surface.
6. Interpret the test result in 15 minutes, do not read the test result after
20 minutes.
Note:
1. Don’t use saliva with blood。
2. If the fluid does not move upward , add 1 ml of drinking water to the
plastic bag with saliva, mix the water and saliva evenly , and then put the
absorbing pad back into the bag to absorb more saliva.

Positive(+): Both of T and C lines appear within15minutes.
Negative(-): C line appears while no T line appeared after 15
minutes.
Invalid: If the C line does not appear, this indicates that the test result is invalid,
and you should retest the specimen with another test device.
LIMITSTIONS
1.COVID -19 Antigen Rapid Test Kit is a preliminary qualitative test, herefore,
neither the quantitative value nor the rate of increase in COVID -19 can be
determined by this test.
2.A negative test result may occur if the antigen concentration in a sample is
below the detection limit of the test. The detection limit of the test was determined
with recombinant SARS-CoV-2 nucleoprotein and is 10 pg/ml.
3.The efficacy of the SARS-CoV-2 antigen test cassette has only been evaluated
by the methods described in this package insert. Changes in these procedures may
alter the performance of the test.
4. False negative results can occur when a sample isinadequately detected,
transported or handled.
5. False results may occur if the samples are tested more than an hour after
sampling. Samples should be tested as soon as possible after sampling.
6. Positive test results did not exclude co-infection with other pathogens.
7. Negative test results are not intended to reveal other viral or bacterial infections
from SARS-CoV-2.
8. Negative results from patients with symptomatic onset after more than seven
days should be treated as a presumption and confirmed with another molecular
assay.2 / 2
9.Ifthe differentiation of specific SARS-CoV-2 strainsis necessary, additional
tests are required in consultation with public or local health authorities.
10. Children may tend to secrete viruses longer than adults, which may lead to
differentsensitivities between adults and children and difficult comparability.
11. This test provides a presumptive diagnosis for COVID -19. A confirmed
COVID -19 diagnosis should only be made by a physician after all clinical and
laboratory findings have been evaluated.
NOTES
1. COVID -19 Antigen Rapid Test Kit is only applicable to Saliva samples.
Blood, serum, plasma, urine, and other samples may cause abnormal results.
If any sample tests positive, please see your local healthcare authority for
further clinical diagnosis and reporting of results.
2. Make sure that the absorbing pad is fully moistened.
3. Positive results can be judged immediately ifC line and T line appear,and
negative results need to spend full 15minutes.
4. The test device is a disposable product and will contain bio hazards after use.
Please properly dispose of the test devices, specimens, and all collection
materials after use.
5. Must use prior to the expiration date on product labeling.
6. If part of the test membrane containing the reagents is out of the test
window, or more than 2 mm of filter paper or latex pad is exposed in the
test window, do not use it because the test results will be invalid. Use a new
test kit instead.
Write your message here and send it to us